CHAPTER 4 Blood, lymphoid tissues and haemopoiesis
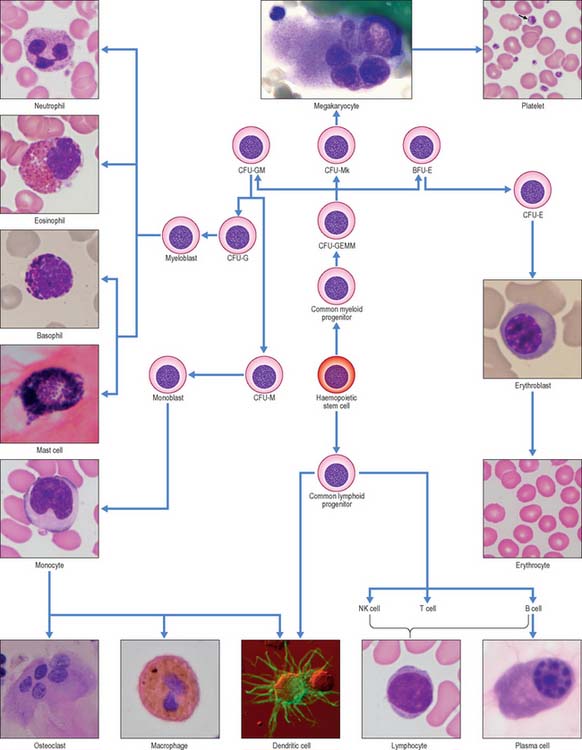
In postnatal life blood cells are formed in the bone marrow. Haemopoiesis produces red cells (erythrocytes), and a wide variety of defensive cells (white blood cells, or leukocytes). The latter include neutrophil, eosinophil and basophil granulocytes, B lymphocytes and monocytes. T lymphocytes develop in the thymus from bone marrow-derived progenitors. Platelets are produced in the bone marrow as cellular fragments of megakaryocytes. Only erythrocytes and platelets are generally confined to the blood vascular system, whereas all leukocytes can leave the circulation and enter extravascular tissues. The numbers of cells doing so increases greatly during inflammation caused by local infections and diseases.
CELLS OF PERIPHERAL BLOOD
BLOOD
Plasma
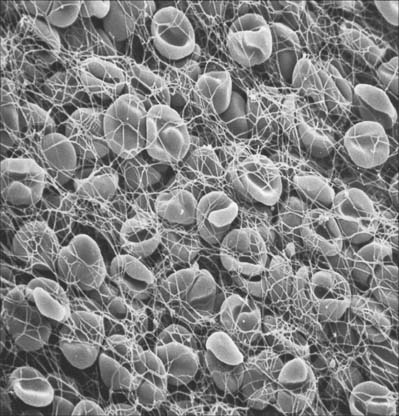
The precipitation of the protein fibrin from plasma to form a clot (Fig. 4.1) is initiated by the release of specific materials from damaged cells and blood platelets in the presence of calcium ions. If blood or plasma samples are allowed to stand, they will separate into a clot and a clear yellowish fluid, the serum. Clot formation is prevented by removal of calcium ions, e.g. by addition of citrate, oxalate or various organic calcium chelators (EDTA, EGTA) to the sample. Heparin is also widely used as an anticlotting agent, because it interferes with another part of the complex series of chemical interactions which lead to fibrin clot formation.
ERYTHROCYTES
Each erythrocyte is a biconcave disc (Fig. 4.1, Fig. 4.2) with a mean diameter in dried smear preparations of 7.1 μm; in fresh preparations the mean diameter is 7.8 μm, decreasing slightly with age. Mature erythrocytes lack nuclei. They are pale red by transmitted light, with paler centres because of their biconcave shape. The properties of their cell coat cause them to adhere to one another by their rims to form loose piles of cells (rouleaux). In normal blood, a few cells assume a shrunken star-like, crenated form: this shape can be reproduced by placing normal biconcave erythrocytes in a hypertonic solution, which causes osmotic shrinkage. In hypotonic solutions erythrocytes take up water and become spherical; they may eventually lyse to release their haemoglobin (haemolysis), leaving red cell ghosts.
Haemoglobin
Haemoglobin (Hb) is a globular protein with a molecular mass of 67 kDa. It consists of globulin molecules bound to haem, an iron-containing porphyrin group. The oxygen-binding power of haemoglobin is provided by the iron atoms of the haem groups, and these are maintained in the ferrous (Fe++) state by the presence of glutathione within the erythrocyte. The haemoglobin molecule is a tetramer, made up of four subunits, each a coiled polypeptide chain holding a single haem group. In normal blood, five types of polypeptide chain can occur, namely; α, β and two β-like polypeptides, γ and δ. A third, β-like η chain is restricted to early fetal development. Each haemoglobin molecule contains two α-chains and two others, so that several combinations, and hence a number of different types of haemoglobin molecule, are possible. For example, haemoglobin A (HbA), which is the major adult class, contains 2α- and 2β-chains; a variant, HbA2 with 2α and 2δ chains, accounts for only 2% of adult haemoglobin. Haemoglobin F (HbF), found in fetal and early postnatal life, consists of 2α- and 2γ-chains. Adult red cells normally contain less than 1% of HbF.
Lifespan
The recognition of effete erythrocytes by macrophages appears to depend in part on the exposure of normally inaccessible parts of membrane proteins, enabling autoantibodies to these erythrocyte senescence antigens to bind to them and flag them for macrophage removal. Red cells are destroyed at the rate of 5 × 1011 cells a day and are normally replaced from the bone marrow (see Fig. 4.12) at the same rate.
LEUKOCYTES
Leukocytes (white blood cells) belong to at least five different categories (see Fig. 4.12), and are distinguishable by their size, nuclear shape and cytoplasmic inclusions. In practice, leukocytes are often divided into two main groups, namely those with prominent stainable cytoplasmic granules, the granulocytes, and those without.
Granulocytes
This group consists of eosinophil granulocytes, with granules which bind acidic dyes such as eosin; basophil granulocytes, with granules which bind basic dyes strongly; and neutrophil granulocytes, with granules which stain only weakly with either type of dye. Granulocytes (Fig. 4.3) all possess irregular or multilobed nuclei and belong to the myeloid series of blood cells (p. 77 see Fig. 4.12).
Basophil granulocytes
Slightly smaller than other granulocytes, basophil granulocytes are 10–14 μm in diameter, and form only 0.5–1% of the total leukocyte population of normal blood, with a count of 25–200/μl. Their distinguishing feature is the presence of large, conspicuous basophilic granules. The nucleus is somewhat irregular or bilobed, and is usually obscured in stained blood smears by the similar colour of the basophilic granules. The granules are membrane-bound vesicles which display a variety of crystalline, lamellar and granular inclusions: they contain heparin, histamine and several other inflammatory agents, and closely resemble those of tissue mast cells (see p. 36). Both basophils and mast cells have high affinity membrane receptors for IgE and are therefore coated with IgE antibody. If this binds to its antigen it triggers degranulation of the cells, producing vasodilation, increased vascular permeability, chemotactic stimuli for other granulocytes, and the symptoms of immediate hypersensitivity, e.g. in allergic rhinitis (hay fever). Despite these similarities, basophils and mast cells develop as separate lineages in the myeloid series, from haemopoietic stem cells in the bone marrow. Evidence from experimental animal models suggests that they are closely related (see Fig. 4.12) but studies on mast cell disorders in humans indicate that their lineages diverge from a more distant ancestral progenitor (Kocabas et al 2005).
Mononuclear leukocytes
Lymphocytes
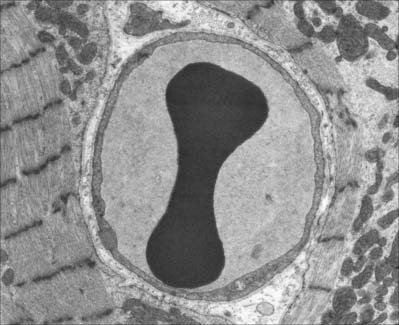
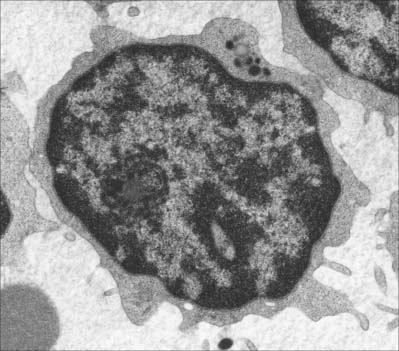
Lymphocytes (Fig. 4.4, see Fig. 4.6, see Fig. 4.12) are the second most numerous type of leukocyte in adulthood, forming 20–30% of the total population (1500–2700/μl of blood). In young children they are the most numerous blood leukocyte. Most circulating lymphocytes are small, 6–8 μm in diameter; a few are medium-sized and have an increased cytoplasmic volume, often in response to antigenic stimulation. Occasionally, cells up to 16 μm are seen in peripheral blood. Lymphocytes, like other leukocytes, are found in extravascular tissues (including lymphoid tissue); however, they are the only white blood cells which return to the circulation. The lifespan of lymphocytes ranges from a few days (short-lived) to many years (long-lived). Long-lived lymphocytes play a significant role in the maintenance of immunological memory.
Blood lymphocytes are a heterogeneous collection mainly of B and T cells and consist of different subsets and different stages of activity and maturity. About 85% of all circulating lymphocytes in normal blood are T cells. Primary immunodeficiency diseases can result from molecular defects in T and B lymphocytes (reviewed in Cunningham-Rundles & Ponda 2005). Included with the lymphocytes, but probably a separate lineage subset, are the natural killer (NK) cells. NK cells most closely resemble large T cells morphologically.
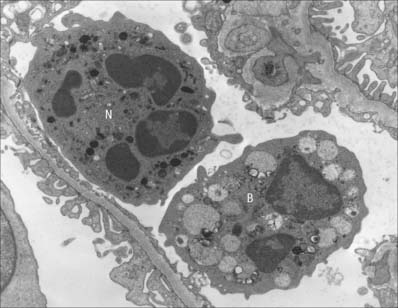
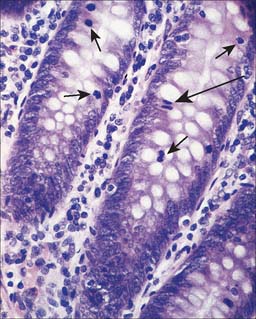
Small lymphocytes (both B and T cells) contain a rounded, densely staining nucleus which is surrounded by a very narrow rim of cytoplasm, barely visible in the light microscope. In the electron microscope (Fig. 4.4), few cytoplasmic organelles can be seen apart from a small number of mitochondria, single ribosomes, sparse profiles of endoplasmic reticulum and occasional lysosomes: these features indicate a low metabolic rate and a quiescent phenotype. However, these cells become motile when they contact solid surfaces, and can pass between endothelial cells to exit from, or re-enter, the vascular system. They migrate extensively within various tissues, including epithelia (Fig. 4.5).
B cells
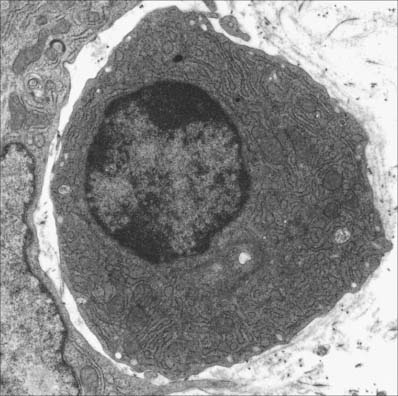
B cells and the plasma cells that develop from them synthesize and secrete antibodies which can specifically recognize and neutralize foreign (non-self) macromolecules (antigens), and can prime various non-lymphocytic cells (e.g. neutrophils, macrophages and dendritic cells) to phagocytose pathogens. B cells differentiate from haemopoietic stem cells in the bone marrow. After deletion of autoreactive cells, the selected B lymphocytes then leave the bone marrow and migrate to peripheral lymphoid sites (e.g. lymph nodes). Here, following stimulation by antigen, they undergo further proliferation and selection, forming germinal centres in the lymphoid tissues. Following this, some B cells differentiate into large basophilic (RNA-rich) plasma cells, either within or outside the lymphoid tissues. Plasma cells produce antibodies in their extensive rough endoplasmic reticulum (Fig. 4.6) and secrete them into the adjacent tissues. They have a prominent pale-staining Golgi complex adjacent to an eccentrically-placed nucleus, typically with peripheral blocks of condensed heterochromatin resembling the numerals of a clock (clock-faced nucleus) (see Fig. 4.12). Other germinal centre B cells develop into long-lived memory cells capable of responding to their specific antigens not only with a more rapid and higher antibody output, but also with an increased antibody affinity compared with the primary response.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree