P. David Rogers
8-1. Key Points
■ Biotechnology, as defined by Merriam-Webster’s Dictionary, is “the manipulation (as through genetic engineering) of living organisms or their components to produce useful usually commercial products (as pest resistant crops, new bacterial strains, or novel pharmaceuticals).”
■ The central dogma of molecular biology is that deoxyribonucleic acid (DNA) encodes ribonucleic acid (RNA), which, in turn, encodes protein.
■ Recombinant DNA technology makes use of several molecular biological tools that allow for the placement of a desired DNA fragment in proximity to other DNA fragments within a DNA molecule for a specific purpose.
■ Cytokines are molecules secreted by cells that orchestrate the immune response. They activate immune cells such as lymphocytes, macrophages, monocytes, and neutrophils.
■ An enzyme is a protein that catalyzes a specific chemical reaction.
■ Hormones are chemical substances transmitted by the bloodstream to cells distant from their physiologic source that impart specific cellular effects.
■ Clotting or blood factors are chemical blood constituents that interact to cause blood coagulation.
■ Vaccines are preparations of antigenic material administered to stimulate the development of antibodies for the purpose of conferring active immunity against a particular pathogen or disease.
■ Subsets of β-lymphocyte clones produce identical antibodies that recognize the same antigen. These identical antibodies are said to be monoclonal. Fusing β-lymphocytes with lymphocyte tumor cells produces a hybridoma that can be cultured in large quantities for the mass production of a given monoclonal antibody.
■ Gene therapy, an application of biotechnology, has great potential therapeutic benefit.
■ Biotechnology has facilitated the development of novel drug delivery strategies, including liposomal technology, immunotoxins, and PEGylation.
■ Pharmacogenomics is the scientific discipline of using genomewide approaches to understand the inherited basis of differences between individual responses to drugs.
■ Single nucleotide polymorphisms are differences in a single nucleotide base occurring at a significant frequency (usually > 5%) within the population. They may result in no change in the encoded amino acid of a codon or a change in the encoded amino acid with no change in the function of the encoded protein. However, when the amino acid substitution attributable to a single nucleotide polymorphism (SNP) results in a phenotypic difference, it may carry clinical relevance.
8-2. Study Guide Checklist
The following topics may guide your study of this subject area:
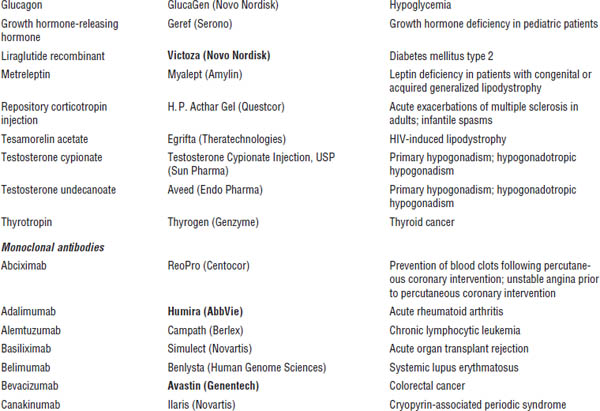
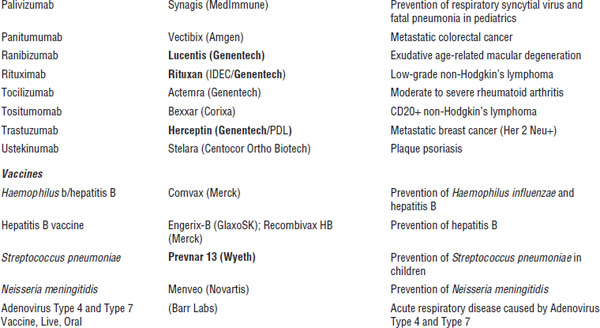
■ Familiarize yourself with marketed biological products (Table 8-1). Focus on products included in the list of “Top 100 drugs.”
■ Gain a general sense of the types of biological products that are currently approved by the U.S. Food and Drug Administration (FDA).
■ Study recombinant technology and the production of vaccines.
Table 8-1. Approved Biological Products
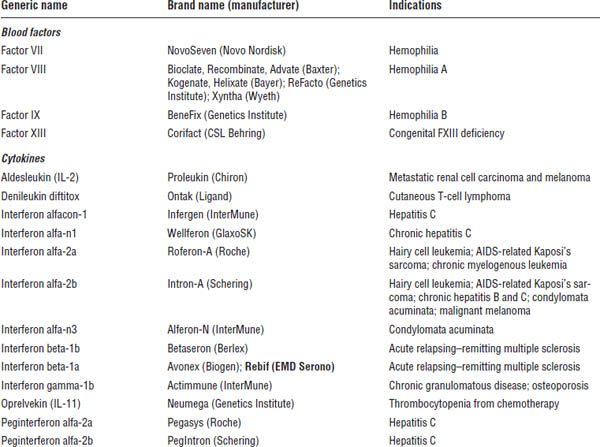


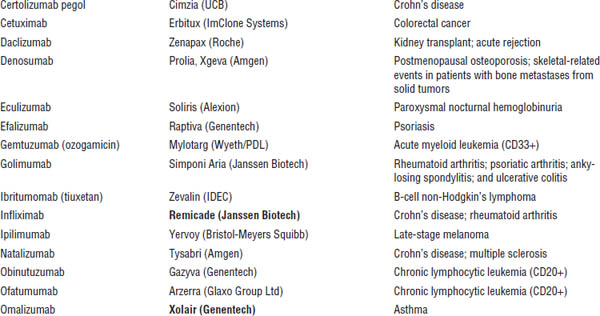

Boldface indicates one of top 100 drugs for 2012 by units sold at retail outlets, www.drugs.com/stats/top100/2012/units.
AIDS, acquired immune deficiency syndrome; BCNU, bis-chlorethylnitrosourea; HIV, human immunodeficiency virus; IL, interleukin; PDGF, platelet-derived growth factor; PTCA, percutaneous transluminal coronary angioplasty.
■ Review the nomenclature of monoclonal antibodies.
■ Familiarize yourself with the concept of pharmacogenomics and major genetic polymorphisms that determine drug response.
8-3. Introduction
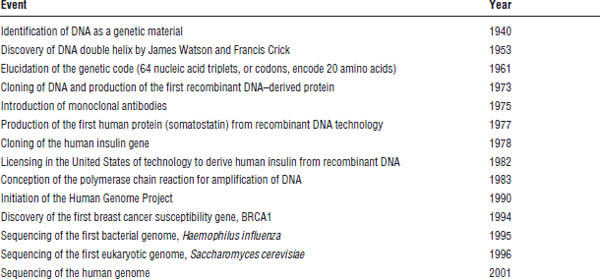
Since the discovery of the DNA (deoxyribonucleic acid) double helix half a century ago, significant use of biotechnology has been made for the improvement of human health (Table 8-2). A number of biological products with therapeutic applications has accompanied these advances. With the arrival of the postgenomic era, the field of pharmacogenomics has emerged and shows great promise to revolutionize the way in which pharmacy and medicine are practiced. This chapter highlights key concepts relevant to the practicing pharmacist in the areas of biotechnology and pharmacogenomics.
Biotechnology has revolutionized the pharmaceutical industry by imparting the ability to mass produce safe and pure versions of chemicals produced naturally in the body. A multitude of disease states have been affected by therapeutic agents derived through biotechnology, including AIDS (acquired immune deficiency syndrome), anemia, cancer, congestive heart failure, cystic fibrosis, diabetes, growth hormone deficiency, hemophilia, hepatitis B and C, and multiple sclerosis, to name a few.
Biotechnology is defined by Merriam-Webster’s Dictionary as “the manipulation (as through genetic engineering) of living organisms or their components to produce useful usually commercial products (as pest resistant crops, new bacterial strains, or novel pharmaceuticals).”
8-4. Key Terms
■ Antibody (immunoglobulin): A protein produced by β-lymphocytes in response to antigen molecules determined to be nonself. Antibodies recognize and bind to antigens, resulting in their inactivation or opsonization for phagocytosis or complement-mediated destruction. A number of immunoglobulin G products have been developed for therapeutic use in various immune disorders.
■ Antigen: A molecule that elicits an antibody-mediated immune response.
■ Bioinformatics: The application of computer sciences and information technology to the management and analysis of biological information.
■ Biotherapy: Any treatment involving the administration of a microorganism or other biologic material.
Table 8-2. Milestones in Biotechnology
■ Clotting factor (blood factor): Chemical blood constituents that interact to cause blood coagulation.
■ Combinatorial chemistry: A drug development strategy that uses nucleic acids and amino acids in various combinations to synthesize vast libraries of oligonucleotide or peptide compounds for high-throughput lead compound screening.
■ Cytokine: An extracellular signaling protein that mediates communication between cells.
■ DNA (deoxyribonucleic acid): A polynucleotide molecule consisting of covalently linked nucleic acids. DNA serves as the genetic material.
■ Enzyme: A protein that catalyzes a chemical reaction.
■ Gene: A region of DNA that encodes a specific RNA (ribonucleic acid) or protein responsible for a specific hereditary characteristic.
■ Gene therapy: Therapeutic technologies that directly target human genes responsible for disease.
■ Genome: The complete set of genetic information for a given organism.
■ Genomics: The scientific discipline of mapping, sequencing, and analyzing genomes. It encompasses structural genomics, functional genomics, and pharmacogenomics.
■ Hormone: A chemical substance imparting specific cellular effects that is transmitted by the bloodstream to cells distant from its physiologic source.
■ Hybridoma: A cell line generated by the fusion of antibody-producing β-lymphocytes with lymphocyte tumor cells for the production of monoclonal antibodies.
■ Interferon: A member of a group of cytokines that prevents viral replication and slows the growth and replication of cancer cells.
■ Interleukin: A member of a group of cytokines involved in orchestration and regulation of the immune response.
■ Liposome: A microscopic, sphere-like lipid droplet that functions as a therapeutic carrier.
■ Monoclonal antibody: An antibody derived from a hybridoma cell line.
■ Pharmacogenomics: The scientific discipline of using genomewide approaches to understand the inherited basis of differences between individuals in the response to drugs. This field is an expansion of the field of pharmacogenetics, which traditionally considered such inherited differences on a gene-by-gene basis.
■ Plasmid: A small, circular, extrachromosomal DNA molecule capable of replication independent of that of the genome.
■ Polymerase chain reaction (PCR): A molecular biologic technique for amplification of specific DNA molecules.
■ Protein: A functional product of a specific gene consisting of amino acids linked together through peptide bonds in a specific sequence.
■ Proteomics: The scientific field of the study of sequencing and analyzing the expression, modification, and function of proteins on a genomewide or global scale.
■ Recombinant DNA (rDNA) technology: The application of DNA molecules derived by joining two DNA molecules from different sources.
■ Restriction endonuclease: An enzyme capable of cleaving a DNA molecule in a site-specific manner.
■ Ribozymes: RNA molecules with intrinsic enzymatic activity.
■ RNA (ribonucleic acid): A polynucleotide molecule consisting of covalently linked ribonucleic acids. Messenger RNA (mRNA) serves as the template for protein synthesis. Transfer RNA (tRNA) serves as the adaptor molecules between amino acids and mRNA during protein synthesis. Ribosomal RNA (rRNA) serves as a component of the ribosome and participates in protein synthesis.
■ Small molecule chemistry: The field of drug development focusing on small organic nucleotide- or peptide-based molecules derived through either combinatorial chemistry or rational drug design.
■ Single nucleotide polymorphism: Common DNA sequence variations among individuals involving a single nucleotide substitution.
■ Vaccine: A preparation of antigenic material administered to stimulate the development of antibodies conferring active immunity against a particular pathogen or disease.
Biological Products
Many U.S. Food and Drug Administration (FDA)–approved biological products are currently on the market, including blood factors, cytokines, enzymes, growth factors, hormones, interferons, monoclonal antibodies, and vaccines. A list of such biological products is provided in Table 8-1.
Gene Expression and Protein Synthesis
Proteins are the major macromolecular component of the cell and are responsible for conducting most of a cell’s biological activity. Proteins consist of a linear polymer of amino acids linked together in a specific sequence. This specific sequence is responsible for a protein’s structure and function. The initial code for the synthesis of a given protein is stored in a gene on a sequence of DNA that is part of a chromosome within the nucleus of a cell.
The central dogma of molecular biology is that DNA encodes RNA, which, in turn, encodes protein. A given amino acid within a protein is encoded by a triplet of nucleic acid base pairs within the gene encoding the protein. This triplet is called a codon. There are 64 codons encoding 20 different amino acids as dictated by the genetic code.
An overview of transcription, translation, and post-translational modification is shown in Figure 8-1.
Recombinant DNA Technology
Recombinant DNA technology uses several molecular biological tools to insert a desired DNA fragment with a specific purpose in proximity to other DNA fragments within a DNA molecule. Most often, a gene encoding a desired protein is isolated through screening of the genomic library or by use of the viral enzyme reverse transcriptase to generate complementary DNA (cDNA) from the mRNA transcript of the gene. Enzymes called restriction endonucleases allow the cleavage of DNA in the plasmid at very specific locations. The gene is then ligated into a vector, such as a plasmid, for gene cloning or for control of the expression of the encoded protein.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree