Biospecimen Banking
Mark Watson
I. SIGNIFICANCE TO PATHOLOGY AND TRANSLATIONAL RESEARCH. National initiatives such as The Cancer Genome Atlas (http://cancergenome.nih.gov) and the 1,000 Genomes Project (http://www.1000genomes.org/) have greatly enhanced the resources available to better understand the molecular and genetic basis of human disease. Technologies such as gene expression profiling, methylation scanning, comparative genome hybridization, and high-throughput resequencing have also made it possible to harness genomic information to identify disease-specific molecular alterations that occur in human tissues. However, to ultimately understand the significance of basic biological findings toward the improved diagnosis and treatment of human disease, primary human biospecimens (i.e., tissue and body fluids) must be available for clinico-genomic correlative studies. Six important principles embody the rationale for biospecimen banks.
A. Availability of large biospecimen cohorts. The number of specimens available must be relatively large to generate statistically significant findings. For example, because of the large number of variables (e.g., gene expression values) generated from DNA microarray experiments compared to the much smaller number of observations (patients), traditional statistical approaches generate a large number of false positive results, that is, genes whose expression appears to classify subpopulations of patients but only does so by mere chance. Validating findings from such whole genome approaches requires large numbers of patient specimens.
B. Accurately diagnosed biospecimens. Several anecdotal examples have illustrated how gene expression profiling can correctly classify specimens that were histologically misidentified by routine clinical pathology (Am J Pathol. 2001;159:1231). However, initial diagnostic mislabeling can seriously impede supervised learning approaches to identify new biomarkers. In addition to issues of diagnostic accuracy, knowledge of the precise cellular makeup of specimens (particularly for heterogeneous tissues) is important to properly interpret both gene expression profile and DNA sequencing data. Techniques such as laser capture microdissection (LCM) can be used to isolate homogeneous cell populations (Acta Histochem. 2007;109:171), but requires considerable technical expertise. An added level of complexity in longitudinal studies is that multiple specimens collected from the same patient need to be accurately coded and tracked to ensure that the intended specimen (e.g., initial presentation vs. relapse) is used for downstream analyses.
C. Availability of specimens with high molecular integrity. Although genomic DNA is relatively stable and unaffected by variables in clinical specimen processing and storage, the same is not true for cellular RNA and protein. Cellular RNA and protein may be degraded in cells ex vivo, if clinical specimens are not rapidly and properly processed and stored (Am J Clin Pathol. 2002;118:733). More insidiously, the mRNA and protein complement of cells may rapidly change as a function of processing time and methodology (J Clin Oncol. 2006;10;24: 3763).
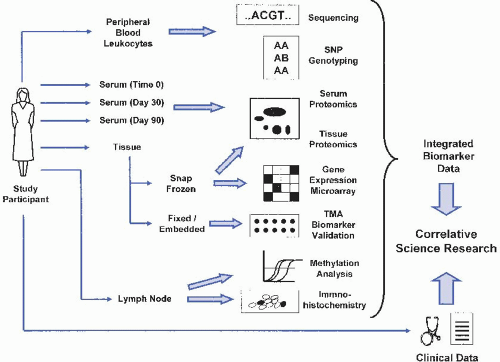
D. Maximal utilization of diverse biospecimens. Because of the number and diversity of potential biomarkers that can be evaluated from banked biospecimens, repositories should contain a wide variety of specimens in a format that can be distributed as widely as possible. For example, preoperative serum
from cancer patients may be useful for identifying tumor-associated serum markers for early detection, while nucleated cell fractions from bone marrow, peripheral blood, or cavity washings from the same patient may be used to evaluate markers for tumor metastasis detection (Figure 62-1). Collected specimens may be rare or small in size, so strategies to efficiently consolidate specimen resources (such as tissue microarrays [TMAs]) or to molecularly amplify limiting amounts of genomic material from needle biopsies, tissue touch preps, or washings are critical to providing a useful specimen resource.
E. Biospecimen annotation. Given the cost and effort associated with next generation sequence analyses of clinical specimens (or proteomic or metabolomic analysis), molecular data associated with human biospecimens is arguably more valuable than the specimen itself. A data system that can accurately track individual samples within large clinical specimen sets and that allows for accurate integration and correlation of sequence, expression, biomarker, and clinical data is obviously crucial. A large and viable specimen resource is only useful if it is linked to complete and accurate clinical data that can be used to substantiate or refute clinical hypotheses. At the same time, increasing concerns for medical record privacy and, in particular, genetic data privacy require proper measures to protect patient confidentiality.
F. Biospecimen custodianship. There have been increasing concerns regarding ownership of tissue specimens (Clin Chem. 2010;56:1675) and the intellectual property that is generated from their use. There is also a continuing need to appropriately honor the intent and privacy of patient donors. This requires the establishment of an unbiased agent who can judiciously administrate and regulate the collection and distribution of specimens.
The biospecimen bank is such an integral part of medical research, and is becoming such an integral part of clinical medicine, that several national and international agencies, such as the National Cancer Institute (NCI), have created dedicated offices and guidelines for bank operation (http://biospecimens. cancer.gov). The principles of biospecimen banking obviously relate to the general practice of pathology and, therefore, it is almost exclusively the pathologist and the pathology department that have sufficient expertise to govern these activities. The development and maintenance of a biospecimen resource involves many policies and processes which are outlined in Figure 62-2, summarized in this chapter, and described in more detail elsewhere (Br J Cancer. 2004;90:1115; Methods Mol Biol. 2010;576:1).
II. BIOSPECIMEN COLLECTION. The types of biospecimens collected and the methods used to process and store them depends greatly on their projected use, although for many specimens the intended use is unknown. Since the cost associated with biospecimen procurement and storage is not insignificant ($20 to $150 per specimen), careful consideration should be given to the scope and focus of the bank’s operation (Clin Transl Sci. 2009;2:172).
A. Defining the scope of operation. The type and number of participants from whom biospecimens are collected depends upon the stated mission and available resources of the biospecimen bank. In some cases, small banks may collect only defined specimens from participants enrolled in specific clinical trials. In other cases, the bank may be disease-based and seek to generically collect all available tissue specimens for a given disease type. When resources or institutional barriers do not permit the creation of a large centralized facility, several small banks may elect to create a federated system where each bank operates independently, but is linked by a common informatics network (Adv Exp Med Biol. 2006;587:65). A biospecimen bank need not be a “bank” at all, but may simply serve to collect, process, deidentify, and immediately distribute biospecimens; the Cooperative Human Tissue Network frequently operates as such a tissue broker (Cancer Epidemiol Biomarkers Prev. 2009;18:1676). A biospecimen bank may also simply exist as a more formal representation of diagnostic paraffin block specimens already available in the pathology department.
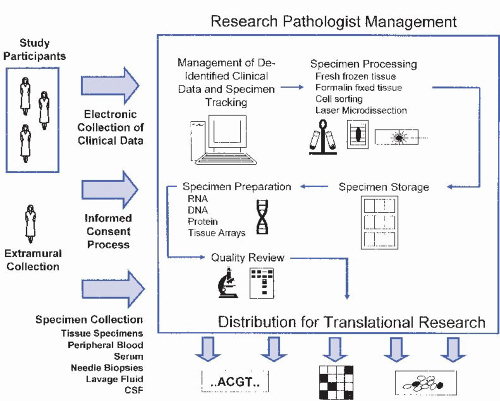
Figure 62.2 Flow diagram of the many processes and resources required to operate a typical full service biospecimen resource
B. Regulatory requirements. Biospecimen banking is going to be a necessary ancillary activity to provide tissue for emerging genomic, proteomic, and metabolomic testing methods. Several regulatory agencies have therefore produced guidelines for clinical biospecimen banks (e.g., www.cap.org) to support direct patient care.
However, many aspects of biospecimen banking are considered a research activity and must conform to regulatory requirements that are different than for a clinical laboratory. Policies have evolved significantly at the national level over the past 5 years, and also vary greatly between states and institutions. Generally, the following points need to be considered with regard to biospecimen-based human subjects’ research.
1. Institutional Review Board (IRB) review. The intended purpose, scope, and policies of the biospecimen bank must be reviewed by the IRB. In some cases, a Certificate of Confidentiality, a document that asserts the right of the tissue bank director (or honest broker) to protect the confidentiality of biospecimen data even under court order, may be required (Genet Test. 2004:8:209).
2. Participant consent. In most cases of prospective biospecimen collection, some form of informed participant consent is required. Explicit informed consent may be waived if it is impossible or impractical to obtain and the risk to the participant is minimal. Rarely, some institutions have ruled that generic language present in a hospital admissions document or surgical consent form provides sufficient consent for biospecimen collection, assuming that the specimens are distributed and utilized in a de-identified or anonymous manner. Generally, however, explicit written consent for biospecimen banking should be obtained from the participant. Many generic templates for the language used in such a document are available (J Clin Pathol. 2006;59:335). Since the main risk to an individual participating in a biospecimen banking program is loss of confidentiality, measures used to protect such confidentiality and the risks associated with this loss are the main risks to convey to the participant. However, when human biospecimens are being used for genomic and disease-predisposition studies, and when research results from biospecimen use generate patentable biomarker inventions, properly documented more specific informed participant consent may be critical.
3. Investigator agreements. In addition to IRB requirements that must be satisfied by the biospecimen bank for the initial collection, coding, and storage of biospecimens, investigators seeking to utilize biospecimens from the bank may need to meet additional regulatory requirements, which usually requires approval of an independent IRB protocol. In addition, investigators may be expected to sign a Data Use Agreement or other investigator agreement that establishes what will or will not be done with distributed biospecimens. If specimens are to be distributed to an investigator outside of the bank’s institution, a material transfer agreement (MTA) may also be necessary.
C. Specimen types
1. Tissues. Collected tissues may be snap-frozen or fixed in a variety of crosslinking or precipitating fixatives. Snap frozen tissue provides the highest quality protein and nucleic acid derivatives, and is often required for genomic or proteomic studies. However, the logistics of frozen tissue collection are difficult and proper storage is relatively expensive. Formalin-fixed, paraffin embedded (FFPE) tissue blocks are easy to obtain, easy to store, and very familiar to any pathology service. The quality of molecular derivatives from such tissues is vastly inferior, although several recent technical advances allow FFPE tissue to be used for the generation of DNA sequence and RNA
expression data (PLoS One. 2011;11;6:e17163; J Mol Diagn. 2011;13: 325). Fixation of tissue in precipitating fixatives such as ethanol, acetone, and others (Mod Pathol. 2001;14:116; Diagn Mol Pathol. 2011;20:52), followed by embedding in low temperature polymers (J Mod Diagn. 1999;1:17), provides tissue with excellent histologic detail and molecular material that is superior to that of traditional FFPE tissue (e-Fig. 62.1).* However, RNA and protein quality still do not match that of material derived from frozen tissue.
2. Blood components. Serum and plasma (often collected at multiple time points throughout a patient’s clinical course) are often banked. They are the preferred biospecimen for proteomic analysis, but when properly aliquoted from multiple patients from multiple time points, can occupy a large amount of storage space. Peripheral blood leukocytes are the ideal source for germline DNA, and can be stored or processed without the need for additional cell separation protocols such as mononuclear cell isolation. Bone marrow may also be banked, particularly for analysis involving hematologic malignancies, other bone marrow dyscrasias, or occult tumor cell detection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree