Fig. 11.1
Immunohistochemistry for COX-2 after 7 days (a) and 21 days (b) post injured muscle of rats. Thick arrow indicates COX-2-positive cells and thin arrow indicates COX-2-negative cells. Immunohistochemistry staining. Scale bar = 50 μm
In Fig. 11.2, we can see also the same time analysis after muscle injury, but the immunomarker at this time was the VEGF antibody. We can note that for this immunostaining there was an inversion of marking periods, i.e., at the early time (7 days), we cannot see specific antibody for VEGF, as expected, but 21 days after the injury (Fig. 11.2b), positive signals of VEGF marker in the muscle during repair are visible, suggesting a greater possibility of blood vessel formation which is one of the main actions of this growth factor.
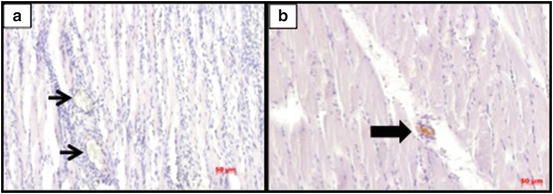

Fig. 11.2
Immunohistochemistry for VEGF after 7 days (a) and 21 days (b) post injured muscle of rats. Thick arrow indicates VEGF-positive cells and thin arrow indicates VEGF-negative cells. Immunohistochemistry staining. Scale bar = 50 μm
Many other processes can be studied using the immunomarkers as a tool. Under a look of great interest are the SCs. Its activation and development to become a mature muscle cell such as during embryonic development, as well as the recapitulation of this process, as in muscle regeneration, have been a goal of several research groups. Knowing which cellular signals and factors affect this signaling is a key to a better understanding of the steps involved in the expression of such cells.
The quiescent SCs were identified by the expression of Pax7, a widely used marker, believed to be ubiquitously expressed in this cell population. The paired box transcription factor (Pax7) was isolated by representational difference analysis as a gene specifically expressed in cultured satellite cell-derived myoblasts. Satellite cells and muscle-derived stem cells represent distinct cell populations. Seale et al. [27] suggest that the induction of Pax7 in muscle-derived stem cells induces satellite cell specification by restricting developmental programs.
Pax7 is required for the maintenance of the postnatal skeletal muscle since the SCs are depleted in the adult Pax7–/– mouse. The results demonstrate Sulf1A re-expression in the regenerating muscle and rapid Sulf1A activation in vitro that precedes asynchronous MyoD activation. It is very interesting because not only an exposure to HGF but also a reduction in Sulf1A levels by neutralizing antibodies induces greatly enhanced satellite cell proliferation and a subpopulation of satellite cell progeny characterized by rapid Pax7 and MyoD downregulation without myogenin activation [28]. To achieve these goals, the authors marked with antibodies specific for Sulf1A, Pax7, and DAPI and overlapping to some of these markers. Furthermore, they performed metabolite markings that are involved with the activation and differentiation of satellite cells, including their molecular signaling. They used diverse time point markings to assess the dynamics of the process of activation and morphological organization of the muscle regeneration by SCs.
In an approach of multiple labels of proteins in the same tissue section, show unequivocal staining of the SC nucleus by MyoD and myogenin. These antibodies also mark the myoblasts and myotubes in the regenerating muscles. These authors developed a multiple marker method, sometimes in serial sections of the tissue, to compare the staining for MyoD, myogenin, c-Met, and DLK1 associated with staining for NCAN or Pax7, which are the reference markers of SCs. MyoD+ and/or myogenin+ nuclei were seen inside the basal lamina of a few dystrophin-negative myofibers, containing Pax7+ and NCAN+, Pax7− and NCAN+ cells, and some macrophage nuclei, as well as in regenerating (NCAN+) myofibers. As expected, MyoD+ and myogenin nuclei were present in fibers under repair. These multiple markers can help to identify the location of the nuclei, whether inside or outside of the basal lamina. This is one of the methods to select the SCs on muscle slides.
Horvathy et al. [29] studied repeated trauma on the muscle and their regeneration when compared with a single trauma. Using BrdU staining for newly divided cells, Hoechst staining for nucleus, and laminin staining, they could differentiate between the intact area of the muscle, the necrotic area (main lesion), and the penumbra area (the secondary lesion). The model used was cryolesion in two time points: 1 and 5 weeks after the last cold lesion. According to the results, not only muscle progenitor cells are found in the site of the lesion but also fibroblasts that can generate fibrosis and reduce the function of muscles. The authors conclude that the repeated trauma does not affect the number of proliferating cells and around 50 % of these cells are incorporated into newly formed myofibers in both single and repeated trauma groups. This indicates that single trauma and polytrauma injury does not impair the proliferating capabilities of a myogenic precursor.
In the same way, the signaling pathway of muscle trauma was analyzed by Toth et al. [30]. They explored the STAT3 signaling mediated by interleukin-6 (IL-6) as a regulator of the satellite cells. The immunohistochemical analysis revealed a progressive increase in the proportion of SCs expressing p-STAT3 with 60 % of all SCs positive for p-STAT3 at 24 h after the provocation of injury. The injury in this case was performed by 300 maximal eccentric contractions of the quadriceps femoris at high speed.
Although conducted in vitro, such experiments can also provide much information and learning experiences. An example of this was accomplished in the work of Wang et al. [31] when evaluating the effects of different frequencies of vibration on the formation of myotubes in C2C12 cells in culture. For this, they used the marker anti-myosin (MF-20), with Alexa Fluor 488 labeled as a secondary antibody and DAPI to mark the nuclei. They found interesting results at day 6 post treatments like the average lengths of myotubes in the 8 and 10 Hz of frequencies are significantly greater than 5 and 0 Hz groups. At day 9, the myotube lengths at all the frequencies (5, 8, and 10 Hz) were bigger than the control group (0 Hz).
IHC as a Clinical Aid for Diagnosis and Treatment Monitoring
In general, when performing experiments in animal models, the main objective is to transfer these technologies for use in diagnosis and treatment for humans. Precisely for this reason, the advances that occur in the use of antibodies in experimental animals (rats and mice in particular) can and should be carefully studied and applied to facilitate the laboratory diagnosis of muscle diseases in human subjects.
Comparative analysis of flow cytometry with IHC was conducted in human muscle samples. The IHC showed to be a powerful method to investigate the number of satellite cells using the immunomarkers NCAM and c-Met. It was reliable and effective for measuring this parameter, but the flow cytometry was faster and independent of the expertise of the investigator involved [32]. They studied the cell cycle in the same investigation using the immunomarker Pax7+, and they found that more information about the kinetics of the activities of the cell was provided when using flow cytometry compared to IHC. However, the authors do not dismiss the IHC as an effective method for this kind of analysis. The use of these kinds of markers can improve the diagnostic process for scientific investigation and in the clinical set.
The clinicians who work with patients with neuromuscular diseases know the importance of immunohistochemistry in the differentiation of dystrophies in humans, especially the recessive forms. It seems that the expression of some modified proteins is quite distinct when comparing the Xp21 dystrophy (Duchenne), Emery–Dreifuss muscular dystrophy, and the limb-girdle dystrophies. Dystrophin is the defective protein in Duchenne muscular dystrophy (DMD) . Therefore, we can look at the differential labeling that is explained by gene deletions that disrupt the reading frame and give rise to the severe DMD phenotype and those that maintain it and produce the milder phenotype of Becker muscular dystrophy. Approximately 95 % of cases follow this dogma but there are several exceptions. For example, the deletion of exons 3–7 is a frameshift deletion that should result in no expression of dystrophin and a severe phenotype, but these cases often have an intermediate phenotype and some dystrophin is localized to sarcolemma [33]. The author shows other potential markers like antibodies to emerin, sarcoglycans, calpain 3, dysferlin, laminin α2, utrophin, and fast and slow isoforms of myosin that can be applied for such investigations.
In a very good review, Matos et al. [6] covered the use of IHC as an aid in surgical and clinical settings to determine the directions of treatment for patients who had biopsies. They discuss from the point of view of the pathologist and of the clinician and the surgeon the relative merits and discriminative aspects of IHC as a diagnostic tool.
The authors emphasize the importance of the method and cover possible applications. Among them may be cited: (1) histogenetic diagnosis of morphologically non-differentiated neoplasms; (2) subtype of neoplasms (e.g., lymphomas); (3) characterization of primary site of malignant neoplasms; (4) research on prognostic factors and therapeutic markers for some diseases; (5) discrimination of benign versus malignant nature of certain cell proliferations and identification of structures, organisms, and materials secreted by cells [6].
In the same review, Matos et al. [6] discuss the limitations and difficulties of IHC. They point out that the experience of the examiner may affect the preparation and interpretation of the results. However, if the procedure is carried out carefully and accurately, it can ensure very good results for later analysis. A wide variety of protocols for standardizing the IHC techniques are proposed to minimize undesirable effects. The Committee of Quality Control in IHC of the French Pathology Society published a report in 1997 demonstrating that two of the main causes of diagnostic mistakes in IHC are the nonemployment of antigen retrieval techniques and the use of amplifying methods with low power. Other renowned international quality programs are the electronic database ImmunoQuery (Immunohistochemistry Literature Database Query System) and the UK NEQAS quality program (United Kingdom National External Quality Assessment Scheme for Immunocytochemistry) [6]. For the diagnosis of the muscle, both for diseases, such as to detect stages of inflammation or tissue repair, it seems really important to exclude bias from the study process to achieve a high degree of assertiveness.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


