Fig. 4.1
Flow cytometric analysis of spermatid-specific thioredoxin 3 (SPTRX3/TXNDC8) in semen of ART patients. The SPTRX3 protein accumulates in the superfluous cytoplasm and nuclear vacuoles of defective spermatozoa. Scatter diagrams of visible light, reflective of the size of individual cells/flow cytometric events in each sample, are in the left column. Each dot represents one cell/event. Normal size spermatozoa cluster in the lower left corner and toward the center of diagram. Small debris is in the extreme lower left; abnormally large spermatozoa and somatic cells cluster toward the right side of the diagram. Histograms of SPTRX3-induced fluorescence are shown on the right. Median (Med) is the median value of SPTRX3-induced fluorescence at which half of the events have higher and half have lower relative fluorescence (no units) of immuno-labeled SPTRX3 protein. Histograms are divided into three marker areas: M1—events representing cellular debris and sperm fragments with very low background fluorescence; M2—events representing mainly normal spermatozoa with background levels of SPTRX3 fluorescence and cells/debris of similar size free of SPTRX3; M3—events representing spermatozoa positive to SPTRX3. Marker area M3 was set differently in some of our previously published studies, resulting in higher cutoff fluorescence values for SPTRX3-positive spermatozoa. Percentages of events within each marker area are shown as %M1–M3. A total of 5,000 events were measured per sample. (a) Reference sample with acceptable WHO sperm parameters. Histograms show normal distribution. Med and %M3 values are low, 74 % of spermatozoa/events fall within marker area M2. (b) Slightly elevated Med and %M3 values are reflective of a shoulder on the right side of histogram, corresponding to the SPTRX3-positive spermatozoa. (c) Distinct secondary peak covers the M3 area, reflected by elevated Med and %M3 values. (d) Sample with normal distribution but with the histogram peak shifted to M3 area, resulting in high median and %M3 value above 50 %. In the absence of positive and negative controls, and without appropriate sample blocking prior to antibody labeling, this type of curve could also be obtained by over-labeling resulting in elevated nonspecific background fluorescence. (e) While the Med and %M3 values are similar to panel (d), the shape of histogram curve is dramatically different, essentially composed of two peaks of equal height and width. (f) Sample with low overall fluorescence corresponding to low Med and %M3 values similar to reference sample in panel (a). However, the shape of the histogram curve is unusually flat and a large number of events positioned toward the right and upper right part of the scatter diagram suggest the presence of large cells that do not express SPTRX3, such as leukocytes. While clearly a contaminant/abnormality, such cells can lower the overall reading of biomarkers associated exclusively with the defective spermatozoa. This issue can be ameliorated by dual analysis with markers of white blood cells. (g) Sample with only a slight increase of Med value, but a very flat histogram curve and an elevated %M3 value. This was a sample with very few spermatozoa which did not allow to measure 5,000 events, as reflected by fewer events seen in the scatter diagram. Such samples are often encountered with oligozoospermia. Due to low sperm concentration, debris and somatic cells likely make up a substantial percentage of measured events. This issue could be mitigated by double labeling with a DNA-specific probe, which would allow for gating of spermatozoa during SPTRX3 labeling
Sperm Flow Cytometry with Vital Stains and Lectins
Mitochondrial membrane potential (MMP) reflects the polarization of mitochondrial membrane and thus the metabolic state/activity of sperm mitochondria. Low MMP is indicative of elevated apoptosis or necrosis in the semen sample [12, 13]. Human sperm MMP measured by vital ratiometric dual-fluorescent probe JC-1 is correlated with sperm motility [14–16]. A recent study of normozoospermic and asthenozoospermic men revealed a correlation between sperm MMP and expression of inner mitochondrial membrane protein prohibitin/PHB [17]. Other fluorometric mitochondrial probes, such as CMX-Ros, DiOC(6)(3), rhodamine 123, and TMRE, can be used as an alternative or complement to JC-1 [18].
Sperm viability obviously has an effect on the fertilizing potential of an ejaculate specimen. Live/dead cell stains are based on differential cell membrane permeability (plasma membrane and nuclear envelope) of fluorescent DNA binding probes. The most common combination is the green fluorescent CYBR-14 probe permeant mainly to live spermatozoa and propidium iodide (PI) excluded from live spermatozoa but easily intercalated in the DNA of the dead ones [19]. While viability can be measured by PI staining alone, it is desirable to include CYBR-14 since different degrees of its exclusion differentiate not only between live and dead spermatozoa but also identify the moribund, dying spermatozoa [20], which is a characteristic similar to the sensitivity of MMP measurement.
Sperm capacitation encompasses the irreversible remodeling of sperm plasma membrane and acquisition of hyperactive motility in preparation for fertilization [21]. If induced prematurely by semen handling, storage, or cryo-damage (cryo-capacitation), capacitation may preclude successful fertilization and eventually lead to sperm death. Since capacitation is accompanied by fluxes/increases in the content of intracellular calcium [22], it can be monitored by flow cytometry with fluorescent Ca-ion reporter dyes such as Fluo-3 or Fluo-4NW [23] and used as a clinical parameter to diagnose male infertility [24]. Besides FC, capacitation status is commonly monitored by epifluorescence microcopy of fluorescent chlortetracycline labeling of spermatozoa [25], but this technique is yet to be translated into a flow cytometric assay.
The sperm acrosome is important for sperm interactions with the oviductal epithelia and oocyte zona pellucida. The structural and functional status of acrosomal membranes, particularly the outer acrosomal membrane, can be affected by capacitation status, acrosome reaction, mechanical damage, or cryo-injury. Sperm acrosomal integrity is evaluated by labeling of live spermatozoa with fluorescently conjugated lectins, glycan binding plant proteins with narrowly defined binding affinity to specific types of sugar residues found on sperm glycoproteins. In particular, the peanut agglutinin (PNA or Arachis hypogea lectin) and the green peas derived Pisum sativum agglutinin (PSA) display high specific affinity toward glycans of sperm acrosomal matrix and have been adapted for FC [26–29]. Thus, only spermatozoa with compromised acrosomal membranes bind PNA and PSA, which can be used in both live spermatozoa and fixed spermatozoa via a dual labeling protocol applying antibodies or DNA stains [30, 31]. Other acrosome binding lectins used for human sperm FC include wheat germ agglutinin (WGA), Ulex europaeus agglutinin (UEA-1, ulex, or common gorse seed lectin), and Concanavalia ensiformis agglutinin (Con-A or common jack bean lectin) [32, 33].
Protein Biomarkers of Sperm Quality
Normal and defective spermatozoa may accumulate certain proteins at differential levels, and they may lack certain other proteins. Whole proteome analyses comparing spermatozoa from fertile donors with male infertility patients revealed a number of such proteins [34]. Based on the observation that proteins such as ubiquitin accumulate on the surface of defective spermatozoa, we proposed the description “negative biomarkers of male fertility and semen quality” [11]. This umbrella term encompasses proteins that are increasingly or exclusively present in defective spermatozoa, often retained from the haploid phase of spermatogenesis occurring in the testis. Proteins such as thioredoxin SPTRX3, discussed below, have a function during the biogenesis of sperm accessory structures but then are degraded within the spermatid cytoplasmic lobe or jettisoned within a residual body. In defective spermatozoa that fail to complete spermatid differentiation, such proteins may be retained in structures containing residual cytosol such as nuclear vacuoles and the retained cytoplasm surrounding the sperm tail connecting piece and midpiece.
Ubiquitin and Ubiquitin-Like Protein Modifiers
Ubiquitin (UBB) is a small chaperone protein that binds covalently to other proteins, most commonly in a tandem fashion giving rise to multi-ubiquitin chains making the ubiquitin-tagged substrate proteins recognizable to the 26S proteasome, which is a proteolytic holoenzyme particle responsible for regulated, substrate-specific protein recycling across the human, animal, and plant proteomes [35]. Such protein modification by this ubiquitination is reversible and has regulatory functions in addition to promoting selective proteolysis. Examination of ubiquitin as a sperm quality biomarker was initiated based on the observation that defective animal spermatozoa become surface-ubiquitinated by an apocrine secretory mechanism that assures high concentration of ubiquitin-proteasome system enzymes and non-conjugated ubiquitin in the epididymis [36, 37]. Additionally, ubiquitinated proteins from spermatid phase can be carried over in the sperm structures or sperm-borne superfluous cytoplasm. While some appear morphologically normal, most ubiquitinated spermatozoa display a variety of morphological defects and they often carry single-stranded, fragmented DNA detectable by dual TUNEL-ubiquitin FC [38]. Ubiquitin is also present in the normal spermatozoa, but the localization, ubiquitin-substrate ligation patterns, and amounts may differ from the defective spermatozoa.
In our early studies, the flow cytometric sperm ubiquitin-tag immunoassay (SUTI) for diagnosis of human male infertility correlated negatively with various conventional semen parameters, as well as with embryo cleavage rate and other embryo-development parameters after IVF and ICSI [39, 40]. Substantial proportion of men from idiopathic infertility couples tend to have elevated sperm ubiquitin content [40], hinting at the potential of SUTI assay to reveal cryptic male infertility in men with acceptable clinical semen parameters. On the opposite end of spectrum, high sperm ubiquitin levels were found in obviously infertile men with heritable stump tail syndrome/fibrous sheath dysplasia [41], in men with abnormal sperm chromatin [42], and in ART patients with self-reported occupational exposure to reprotoxic solvents [43]. In proteomic analysis, proteins related to ubiquitin-proteasome system were abnormally expressed in infertile men with high DNA fragmentation index determined by flow cytometry [34]. Contrary to elevated defective sperm surface ubiquitination, the flow cytometric measurement of the ubiquitin content intrinsic to normal spermatozoa revealed positive correlation with fertilization rate by ICSI, while no such relationship was observed for simultaneously assessed sperm protamination [44]. The sperm content of “properly” ubiquitinated normal spermatozoa can be increased by sperm gradient purification [45]. In some studies, only certain measures of sperm surface ubiquitination, such as median ubiquitin-induced fluorescence, showed negative correlation with semen parameters, while percentages of high-ubiquitin spermatozoa did not correlate with semen quality, or with various markers of apoptosis [46]. Others determined that ubiquitin was mainly associated with anuclear bodies present in semen, rather than with spermatozoa, but based on images shown, one could suspect that the immunolabeling of the examined samples was not done on properly preserved samples by strictly following published protocols. Such reports may be misleading but still put emphasis on the necessity of proper quality control of sperm immunolabeling prior to flow cytometry [47, 48]. Alternatively, these seemingly conflicting observations could simply mean that in patients with high content of semen contaminants, the measurement of surface ubiquitination unique to spermatozoa simply reveals the ratio of spermatozoa to contaminating somatic cells and residual bodies, and could in fact have a positive correlation with semen quality. Some of the issues associated with the specificity of anti-ubiquitin antibodies and other antibodies for sperm FC and the potential of immunocytochemical detection for false-negative results have been addressed by developing a very simple, single-step detection of stress-associated ubiquitinated protein aggregates, the aggresomes, using the ProteoStat aggresome detection kit originally developed for somatic cells [8].
Ubiquitin-like protein modifiers are structurally and functionally related to ubiquitin and may be involved both in selective protein recycling and in the regulation of substrate protein function. Similar to ubiquitin, the covalent ligation of these modifiers to substrate proteins requires activating and conjugating enzymes and substrate-specific protein ligases. Small ubiquitin-related modifier SUMO1 [49, 50] and its close relatives SUMO-2, 3, and 4 regulate the functions of varied substrate proteins in either reversible or irreversible manner. Similar to increased protein ubiquitination, excessive protein sumoylation by SUMO1 and SUMO2/3 was reported in infertile men and coincided with ubiquitination of several sperm proteins that appeared to be both ubiquitinated and sumoylated [51]. Sperm SUMO1 content correlated negatively with sperm motility in asthenozoospermic but not in normozoospermic men [52]. The presence of other ubiquitin-like modifiers (NEDD4/8, ISG15) in human spermatozoa is yet to be investigated.
Testis-Specific Thioredoxins
Thioredoxin family proteins are involved in the regulation of cellular redox potential, thus affecting protein folding and a variety of cellular functions. There are three thioredoxins uniquely expressed in male germ line of mammals [53]. Among them, the thioredoxin domain-containing 8 (TXNDC8), commonly described as sperm/spermatid-specific thioredoxin 3 (SPTRX3), has been found to accumulate in defective human spermatozoa. Early during spermiogenesis, SPTRX3 is detectable in the pro-acrosomic granule of round spermatids, suggesting involvement in acrosomal biogenesis [54]. While undetectable in fully differentiated normal spermatozoa of humans and other mammals [54], SPTRX3 uniquely carries over into the nuclear vacuoles and superfluous midpiece cytoplasm of defective human spermatozoa [55] (Fig. 4.2). We have found that sperm levels of SPTRX3 correlate negatively with conventional semen parameters and pregnancy outcomes of both IVF and ICSI couples [56]. Among 239 ART couples, only 9.2 % got pregnant if the male partner had >15 % SPTRX3-positive spermatozoa measured by flow cytometry, vs. 41.2 % pregnant couples in which men had less than 5 % SPTRX3-positive spermatozoa. Thus, men with >15 % of SPTRX3-positive spermatozoa had their chance of fathering children by ART reduced by nearly two-thirds [56]. Our yet to be published trials also indicate that low SPTRX3 content significantly increases the likelihood of multiple pregnancy after multi-embryo transfer.
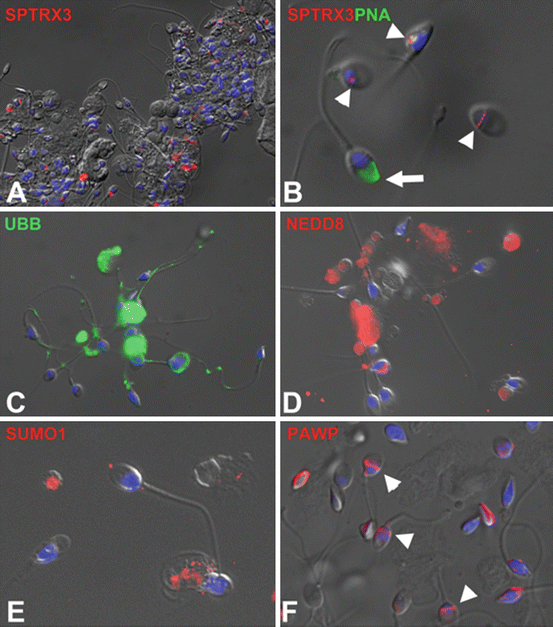

Fig. 4.2
Immunofluorescence localization of sperm quality biomarkers in the spermatozoa of male infertility patients. Sperm nuclear DNA in all panels was counterstained with DAPI (blue) and the epifluorescence images were superimposed over parfocal differential interference contrast (DIC) light images. (a) Spermatid-specific thioredoxin 3 (SPTRX3; red) is abundant in the redundant cytoplasm of defective human spermatozoa. (b) Retention of SPTRX3 (red) in small nuclear vacuoles of defective spermatozoa lacking acrosomes (arrowheads; acrosomes were counterstained green with lectin PNA—arrow). (c) Proteolysis-promoting small protein modifier ubiquitin (UBB; green) is found on the surface of defective spermatozoa and in the interior of anucleate residual bodies present in patients’ semen. (d) Ubiquitin-like protein modifier NEDD8 (red) is localized predominantly to anucleate bodies and superfluous sperm cytoplasm. (e) Ubiquitin-like protein modifier SUMO1 (red) is associated with superfluous cytoplasm of defective spermatozoa (lower left corner). (f) Post-acrosomal WW-domain binding signaling protein PAWP (red) is found in the post-acrosomal sheaths of normal spermatozoa (arrowheads) but may be ectopically localized or missing from defective spermatozoa
The Post-Acrosomal WW-Domain Binding Protein PAWP
(HUGO name WW-domain binding protein N-terminal like/WBP2NL) is an evolutionarily conserved, male germ line-specific signaling protein located in the post-acrosomal sheath (PAS) of mammalian spermatozoa [57, 58] (Fig. 4.2). While the downstream elements of PAWP-regulated signaling pathways in the oocyte remain to be characterized, it has been shown that the injection of PAWP cRNA or recombinant protein induces calcium oscillations identical to those observed during oocyte activation by the fertilizing spermatozoon in human and animal spermatozoa, respectively [59, 60]. Similarly, PAWP sperm phenotypes and semen content of PAWP protein determined by conventional or ImageStream flow cytometry correlate with sperm parameters and fertility in both humans and bovines [8, 61]. The FC sperm content of PAWP in men from ART couples did not correlate with conventional semen parameters or DNA-fragmentation index, but was positively associated with fertilization success and pre-implantation embryo development after ICSI [61]. Given its consistent multi-species validation, PAWP-specific probes are being developed for routine FC use in andrology laboratories. Because of distinct, easy-to-assess localization to PAS of normal spermatozoa and ectopic localization patterns in defective spermatozoa, such probes will also be suitable for light-microscopic evaluation.
The Platelet-Activating Factor Receptor
(PAFR) is a G-protein-coupled receptor-like, rhodopsin-related protein receptor for the pathology-related PAF phospholipid [62]. Based on immunofluorescence and transcript profile, Pafr gene expression and PAFR protein distribution are altered in abnormal human spermatozoa [63, 64]. To our knowledge, PAFR FC has not been conducted in humans, while our earlier study details the relationship between PAFR and sperm quality in bulls entering artificial insemination service [65], suggesting that translation to clinical use may be possible and useful.
White Blood Cell (WBC) and Immune Response Markers
The WBC frequently contaminate semen of infertile men, and sperm FC using biomarkers related to WBC surface antigens (e.g., cluster of differentiation/CD glycoproteins), immune response, and autoimmune infertility are of interest to ART practitioners. Thus, FC can be used to directly identify and quantitate WBC types in semen [66], to simultaneously assess sperm and leukocyte count and sperm apoptotic markers [67, 68], and to measure reactive oxygen species (ROS) production by WBC (main ROS source in semen) and other seminal somatic cells [69]. Exposure of normal spermatozoa to pro-inflammatory interleukins increases sperm DNA fragmentation evaluated by TUNEL-FC [70]. By FC, mast cell counts correlate positively with sperm-bound immunoglobulin IgA in ART men [71], and the CD16-positive lymphocytes and gamma delta receptor-positive T lymphocytes are elevated in autoimmune-infertile men with antisperm antibodies in semen [72, 73]. However, the influence of total semen WBC and individual WBC types on ART outcomes is unclear at present [74], partly because WBC type-specific records are commonly unavailable for ART couples and WBC are also present in the semen of fertile men. It remains to be determined if the content of any particular WBC type in semen correlates with SAB or multiple pregnancies after ART. In addition to anti-sperm antibodies on the sperm surface, immunomodulatory antigens may reflect sperm quality. We reported that the immunoregulatory human sperm glycoproteins decorated with branched, bi-antennary Lewis(x) and Lewis(y) glycans are present in normal sperm acrosome but also detected in the superfluous cytoplasm in defective spermatozoa [75]. While this study employed anti-Lewis antibodies, lectins with appropriate affinities for Lewis glycans could be adapted for sperm FC.
Sperm Protamination
The protamines are sperm-specific, cysteine-rich DNA-binding proteins responsible for hyper-condensation of sperm chromatin following histone-protamine exchange during spermatid elongation in the testis. Human spermatozoa contain both known mammalian protamines, PRM1 and PRM2 [76], as well as residual somatic cell-type histones. Aberrant sperm protamination is associated with human male infertility and correlates with ART embryo development [77]. Consequently, various diagnostic assays can be used to assess human sperm protamination by the quantification of individual protamine types, or by PRM1:PRM2 or protamine:histone ratio [78, 79]. While protamination lends itself to quantification by FC (e.g., chromomycin A3 test [80]), most diagnostic approaches rely on indirect assessment of protamination via flow cytometric chromatin structure/DNA integrity tests [81], as will be discussed next.
DNA Fragmentation, Apoptosis, and Chromatin Structure-Based Tests (TUNEL, Annexin, SCSA)
Sperm DNA integrity and proper chromatin packaging have direct effect on both fertilization and post-fertilization embryo development and maintenance of pregnancy [82, 83]. Some spermatozoa with abnormal chromatin and thus enlarged macrocephalic heads may not be able to reach the oocyte or penetrate its vestments, while morphologically normal motile spermatozoa delivering fragmented DNA to oocytes may give rise to embryos destined for apoptosis due to irreparable DNA damage within paternal genome [3]. The sperm chromatin structure assay (SCSA) is considered by some to be reflective of sperm protamination, while the most direct association may be with DNA fragmentation. To clinicians involved with fertility diagnostics and therapeutic management of couples, DNA fragmentation assessment is widely accepted as valuable. As an adjunct to traditional analyses, routine use of DNA fragmentation can streamline the evaluation process, triage to IVF/ICSI sooner in some couples, as well as diagnose “qualitative” sperm issues that otherwise are undetected by conventional semen analysis. The SCSA is based on the intercalation of metachromatic dye acridine orange with light emission wavelength shifting from green to red fluorescence when bound to single-stranded DNA [84]. As an added benefit, SCSA output can be analyzed to quantitate spermatids and various somatic cells contaminating human semen. The SCSA results are expressed as DNA fragmentation index (DFI) and high DNA stainability (HDS) value [85]. There are many convincing studies showing SCSA correlation with conventional semen parameters and embryo development after ART [3, 85–87]. Even couples with acceptable basic semen parameters may benefit from SCSA before the decision is made to treat by intrauterine insemination [88]. Importantly, several recent studies show the association of high DFI/DNA fragmentation with SAB and multiple births. Relationship between high DFI/HDS and miscarriage after ART has been recorded at varied threshold levels in ART couples [3, 89–91]. Besides significant correlation with SAB, a meta-analysis of 233 couples evaluated by SCSA reported a significantly lower average DFI in couples that had triplets after multiple embryo transfer [5]. Alternative to SCSA, the fluorescent terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) of single-stranded DNA is indicative of increased DNA damage in couples experiencing sporadic or recurrent pregnancy loss [92, 93] and can be adapted for sperm FC [38].
Various markers associated with pathways regulating programmed cell death, apoptosis, have been detected in human spermatozoa [94] and correlated with ART outcomes [95]. Among them, Annexin V is most commonly targeted for defective human sperm identification and removal from sperm samples prepared for ART [96]. The Annexin V assay adapted for FC showed relationship with human sperm mitochondrial membrane potential [97], sperm concentration and motility [98], sperm viability and DNA methylation status [99], advanced male age [100], and sperm cryo-damage [101].
Troubleshooting of Human Sperm Flow Cytometry
Contrary to most mammals, human semen contains abundant cellular debris that requires accurate gating of spermatozoa during FC. Approximate separation can be achieved by gating off the sperm-sized flow cytometric events in scatter diagram (see Fig. 4.1). Such gating will exclude large cells such as leukocytes and small FC events such as cellular debris and contaminants that may be present in reagents used for sperm labeling. However, at the same time, abnormally large spermatozoa could be excluded along with leukocytes and sperm fragments which are also informative of sperm quality and may carry the assessed biomarker molecule. Furthermore, anucleate semen contaminants such as residual bodies could be inadvertently included in sperm analysis if they are sized similarly to spermatozoa. Consequently, a more reliable method is counterstaining of the whole sample with a fluorescent DNA probe such as propidium iodide (PI) for fixed spermatozoa or Hoechst 33342 for non-fixed samples, which reliably distinguishes between spermatozoa and somatic cells based on stoichiometry of DNA content and probe fluorescence intensity, and can be combined both with antibody/lectin labeling and with some of the vital fluorescent probes. The extrapolation between sperm phenotype and biomarker quantity is challenging in conventional FC but easily more addressed by using the ImageStream instrument which combines the high-throughput and fluorometric capabilities of a flow cytometer with multichannel imaging capability of an epifluorescence microscope. ImageStream instrument thus eliminates extrapolation between microscope and cytometer and allows for direct, simultaneous evaluation of individual spermatozoons’ morphology and biomarker fluorescence intensity and localization/distribution pattern [8, 55].
Sample processing quality control is important for all fluorescent probes but particularly important for antibodies. Antibodies selected for immunolabeling of spermatozoa should be carefully validated by Western blotting (WB) for their specificity for the target protein, for their suitability for immunocytochemical procedures, and for their ability to detect the target protein in situ by epifluorescence microscopy as opposed in a denatured electrophoretically resolved sperm protein extract by WB. Batch variability should be considered, particularly for polyclonal antibodies produced by bleeding of immunized animals. Proper titers of secondary antibody conjugates should be determined to minimize background fluorescence. Inclusion of both positive and negative controls is paramount to immunolabeling accuracy. In cases when immunolabeling follows a previously validated, published protocol, care should be taken to source antibodies from the specified manufacturers and to obtain antibodies with catalog numbers identical to the ones published. In many cases, multiple manufacturers offer antibodies of varied quality and specificity. Not adhering to the validated antibody and protocol may produce conflicting results, as discussed for ubiquitin. For any fluorescent probe, quality control of every flow cytometric trial by randomly sampling and examining processed sperm batches under epifluorescence microscope prior to flow cytometric analysis is crucial.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


