chapter 12 Biological membranes and transport
 Because the cell membrane acts as a selective barrier, some molecules need to be transported across the membrane.
Because the cell membrane acts as a selective barrier, some molecules need to be transported across the membrane. Passive transport refers to the movement of molecules from one side of the membrane to the other down a concentration gradient.
Passive transport refers to the movement of molecules from one side of the membrane to the other down a concentration gradient.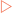 Active transport refers to the movement of molecules from one side of the membrane to another against a concentration gradient.
Active transport refers to the movement of molecules from one side of the membrane to another against a concentration gradient. Specialised transporter proteins are involved in the active transport of substances across the plasma membrane.
Specialised transporter proteins are involved in the active transport of substances across the plasma membrane. The movement of large molecules across the membrane involves gross changes in membrane structure—endocytosis and exocytosis.
The movement of large molecules across the membrane involves gross changes in membrane structure—endocytosis and exocytosis.All cells are surrounded by a phospholipid bilayer membrane, which serves as a barrier to many different molecules. The membrane acts as a selective barrier, allowing the cell to maintain an internal environment that, in many cases, differs significantly from its surroundings. The development of a semi-permeable membrane, which allows some materials to pass while restricting the movement of other molecules, was a major step in the evolution of the cell. All of the biochemical processes described thus far require molecules and ions to be moved across the cell membrane, or membrane transport. This chapter focuses on moving materials into and out of the cell. Transport can occur passively—through diffusion or osmosis—or actively.
The structure of biomembranes
Biological membranes are composed of an aggregate of lipid molecules and associated proteins which form a stable barrier between two aqueous environments— the inside of the cells and extracellular environment (Fig 12-1). As discussed in Chapter 4, many types of lipid molecules are amphipathic, or have both hydrophilic and hydrophobic regions. The fatty acids contained in the molecules that make up the plasma and nuclear membranes of cells in the human body have hydrophilic heads, which are exposed on the outer surfaces of the bilayer, and hydrophobic tails that point inwards away from the aqueous extracellular fluid. This interaction of hydrophobic tails means that phospholipid bilayers form spontaneously, without a requirement for energy input.
Lipids form the structural framework of the membrane, but the membrane also contains a variety of different proteins, which make up about 50% of its weight, distributed throughout the membrane. In 1972, Jonathan Singer and Garth Nicolson proposed a model to describe the arrangement of proteins in the phospholipid bilayer. Their fluid mosaic model proposes that the membrane is a two-dimensional fluid structure in which the lipids and integral proteins are arranged in a mosaic manner (Fig 12-2). The proteins can be thought of as icebergs floating in the sea of the phospholipid bilayer. Singer and Nicolson described two types of membrane-associated proteins, peripheral and integral membrane proteins. Peripheral membrane proteins are those that are not embedded in the membrane, but are indirectly associated with it through interactions with integral membrane proteins and lipid head groups. Members of the integral membrane class of proteins are partially inserted into the membrane itself. A subset of integral membrane proteins is transmembrane proteins that span the membrane, with portions exposed to both the interior and the exterior of the cell. Most transmembrane proteins have α-helical domains of 20–25 hydrophobic amino acids that are embedded in the hydrophobic portion of the phospholipid bilayer. Many are also modified with carbohydrate groups that are exposed on the exterior surface of the cell, making them glycoproteins. There are also a number of carbohydrate-containing glycolipids present in the membrane. These glycolipids are usually oriented so that the carbohydrate group is exposed on the exterior of the plasma membrane. These glycolipids form a carbohydrate coat known as the glycocalyx, which serves to protect the cell surface and to enhance certain cell–cell interactions.
The structure of the phospholipid bilayer determines which molecules can easily pass through it. Any molecule that crosses the bilayer must move through the hydrophobic core in the centre of the bilayer away from the surrounding aqueous environment. Because the interior of the bilayer is composed of hydrophobic fatty acid chains, the membrane is impermeable to water-soluble molecules, including most biological molecules and ions in solution. Thus only a few hydrophobic (nonpolar) molecules such as hydrocarbons, carbon dioxide and oxygen can dissolve in the phospholipid bilayer and cross the membrane without assistance. This means that the nonpolar molecules can readily transit the phospholipid bilayer whereas polar ones cannot. The only major exception to this rule is water, which has surprisingly high permeability for its polar nature. Other substances, including the nutrients required for cell metabolism, require assistance to cross the membrane. Understanding the nature of this assisted movement of molecules across the cell membrane demands a discussion of transport.