Chapter 12 Biological Membranes
Membranes consist of lipid and protein
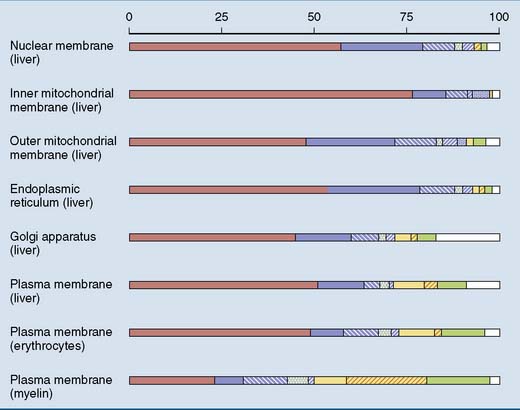
Membranes contain proteins as well as lipids. Lipids form the structural backbone of the membrane, and proteins are in charge of specific functions. These functions include enzymatic activities, regulated transport, ion permeability and excitability, contact with structural proteins, and transmission of physiological signals. Therefore the protein/lipid ratio is highest in membranes with high metabolic activity, such as the inner mitochondrial membrane (Fig. 12.1).
Phosphoglycerides are the most abundant membrane lipids
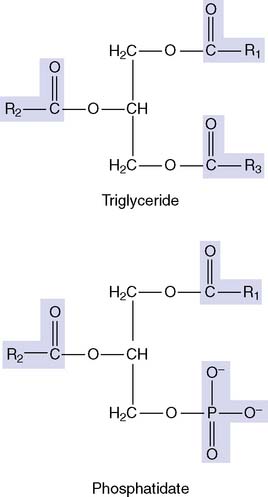
Phosphoglycerides account for more than half of all lipids in most membranes (see Fig. 12.1). Their parent compound is phosphatidic acid, or phosphatidate. It looks similar to a triglyceride but with the third fatty acid of the triglyceride replaced by phosphate:
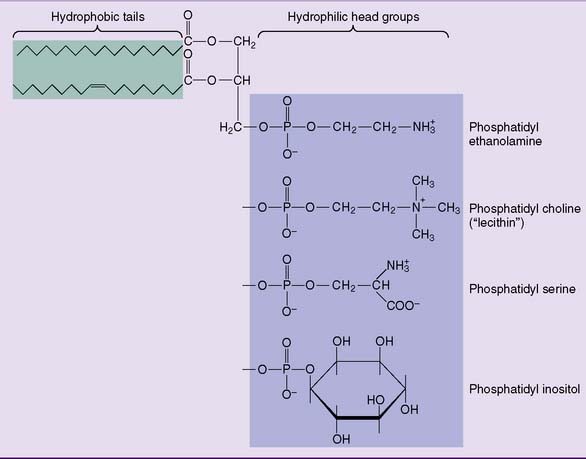
The major membrane phosphoglycerides have a second alcohol bound to the phosphate group in phosphatidic acid, and they are named as derivatives of phosphatidic acid (phosphatidyl-) (Fig. 12.2).
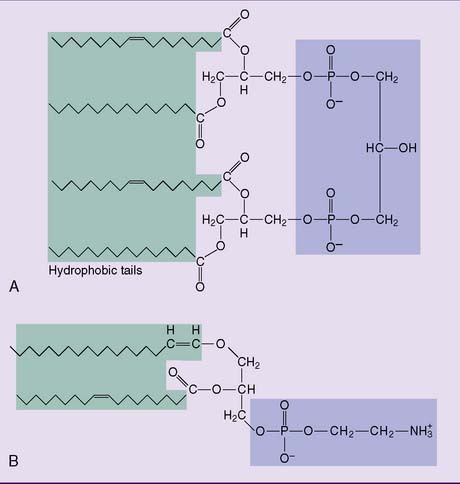
Two less common phosphoglycerides are shown in Figure 12.3. Cardiolipin (diphosphatidylglycerol) is common only in the inner mitochondrial membrane. The widespread plasmalogens, usually with ethanolamine in their head group, are defined by the presence of an α-β unsaturated fatty alcohol, rather than a fatty acid residue, in position 1. In addition to their function as membrane lipids, phospholipids can play other specialized roles in the body (Clinical Example 12.1).
CLINICAL EXAMPLE 12.1: Respiratory Distress Syndrome
The type II alveolar cells in the lungs secrete lung surfactant, which is a mix of lipid and protein with dipalmitoyl phosphatidylcholine (dipalmitoyl lecithin) as its main component. Dipalmitoyl phosphatidylcholine reduces the surface tension by forming a monolayer on the thin fluid film that lines the alveolar walls (see Fig. 12.6D). Without it, the alveoli collapse and breathing becomes difficult.
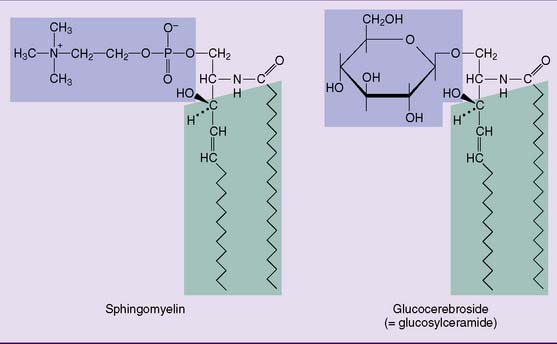
Most sphingolipids are glycolipids
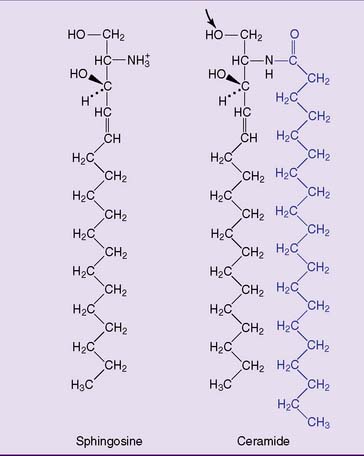
Sphingosine is an 18-carbon amino alcohol with hydroxyl groups at carbons 1 and 3, an amino group at carbon 2, and a long hydrocarbon tail. Ceramide consists of sphingosine and a long-chain (C-18 to C-24) fatty acid bound to the amino group of sphingosine by an amide bond (Fig. 12.4).
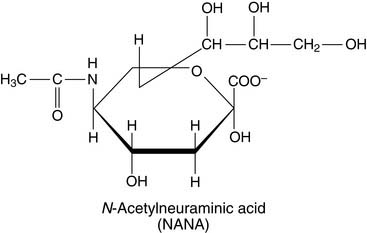
Sphingomyelin (Fig. 12.5), which has the same head group as phosphatidylcholine in Figure 12.2, is the only important phosphosphingolipid. All other sphingolipids are glycolipids. The most complex glycosphingolipids are the gangliosides. They contain between one and four residues of the acidic sugar derivative N-acetylneuraminic acid (NANA) in terminal positions of their oligosaccharide chain:
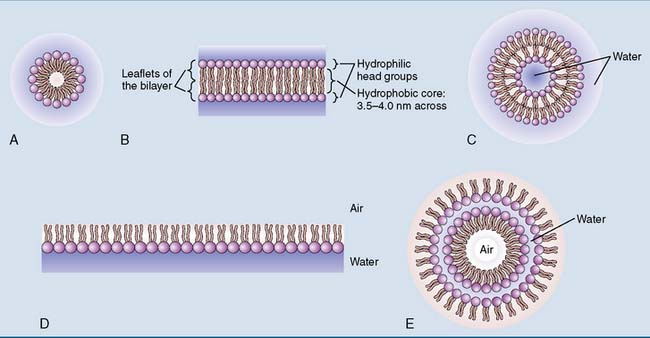
Membrane lipids form a bilayer
The hydrophilic head groups of the membrane lipids interact with water, whereas the hydrophobic tails avoid water. Rather than dissolving in water as individual molecules, the membrane lipids form noncovalent aggregates (Fig. 12.6).
The lipid bilayer is a two-dimensional fluid
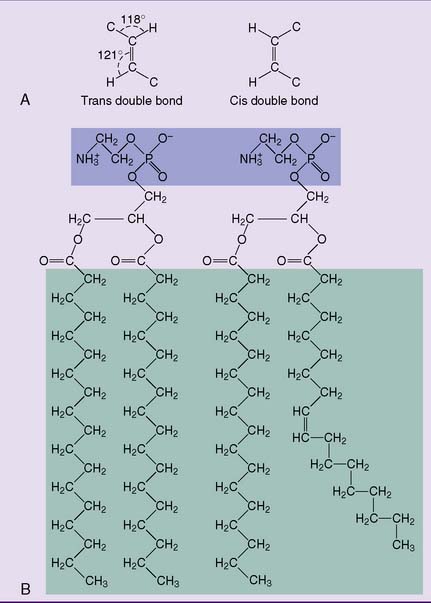
Long, saturated fatty acid chains in the membrane lipids make the membrane more rigid because they align themselves in parallel, forming multiple van der Waals interactions. Unsaturated fatty acids destabilize this orderly alignment because their cis double bonds introduce kinks in the hydrocarbon chain (Fig. 12.7). Therefore a high content of unsaturated fatty acid residues makes the membrane more fluid.
 , Protein;
, Protein;  , phosphatidyl choline;
, phosphatidyl choline;  , phosphatidyl ethanolamine;
, phosphatidyl ethanolamine;  , phosphatidyl serine;
, phosphatidyl serine;  , phosphatidyl inositol;
, phosphatidyl inositol;  , cardiolipin;
, cardiolipin;  , sphingomyelin;
, sphingomyelin;  , glycolipids;
, glycolipids;  , cholesterol;
, cholesterol;  , others.
, others.