Objectives
- Understand the physiologic functions of bile as a route for excretion and in aiding in the digestion and absorption of dietary lipids
- Understand how bile acids are formed from cholesterol, how they are modified during gut passage, and their role in driving bile secretion
- Describe the mechanisms that regulate the rate of conversion of cholesterol into bile acids
- Describe the major biliary lipids and how they are transported into the canaliculus
- Describe how the composition of bile is modified as the bile moves through the biliary ductules
- Define the cellular transport mechanisms that render bile alkaline
- Understand the consequences of a failure to secrete bile, and conditions that cause this problem
Basic Principles of Biliary Excretion and Secretion
 The liver fulfills its excretory function by producing bile, a lipid-rich solution designed to promote the elimination of hydrophobic solutes. Bile consists of a micellar solution in which bile acids, products of hepatocytes produced by the metabolism of cholesterol, form mixed micelles with phosphatidylcholine. These mixed micelles solubilize molecules that would otherwise have minimal aqueous solubility, such as cholesterol itself and a variety of xenobiotics. In addition to its role in providing for excretion of hydrophobic waste products, bile also plays an important role in the digestion and absorption of lipids ingested in the diet. Bile acids form mixed micelles with the products of lipid digestion, increasing the rate at which they can diffuse across the aqueous environment of the gastrointestinal lumen. While bile acids are not essential for the uptake of most fatty acids, which have appreciable aqueous solubility, they do markedly increase the efficiency of this process. On the other hand, insoluble dietary lipids, such as saturated long-chain fatty acids and fat-soluble vitamins, are almost entirely dependent on micellar solubilization for absorption. In patients suffering from cholestasis, deficiencies of fat soluble vitamins can occur. Bile acids also influence bacterial populations in the gut, both by being (in micellar form) directly antimicrobial, and by inducing the expression of genes that protect against bacterial overgrowth.
The liver fulfills its excretory function by producing bile, a lipid-rich solution designed to promote the elimination of hydrophobic solutes. Bile consists of a micellar solution in which bile acids, products of hepatocytes produced by the metabolism of cholesterol, form mixed micelles with phosphatidylcholine. These mixed micelles solubilize molecules that would otherwise have minimal aqueous solubility, such as cholesterol itself and a variety of xenobiotics. In addition to its role in providing for excretion of hydrophobic waste products, bile also plays an important role in the digestion and absorption of lipids ingested in the diet. Bile acids form mixed micelles with the products of lipid digestion, increasing the rate at which they can diffuse across the aqueous environment of the gastrointestinal lumen. While bile acids are not essential for the uptake of most fatty acids, which have appreciable aqueous solubility, they do markedly increase the efficiency of this process. On the other hand, insoluble dietary lipids, such as saturated long-chain fatty acids and fat-soluble vitamins, are almost entirely dependent on micellar solubilization for absorption. In patients suffering from cholestasis, deficiencies of fat soluble vitamins can occur. Bile acids also influence bacterial populations in the gut, both by being (in micellar form) directly antimicrobial, and by inducing the expression of genes that protect against bacterial overgrowth.
Bile Acid Metabolism
 Bile secretion in the liver is driven primarily by the active, ATP-dependent efflux of conjugated bile acids out of the hepatocyte into the canaliculus. In some animals, there is also a variable component of bile acid-independent bile flow, although the solutes that drive this secretion are not fully understood. But in humans, canalicular bile flow is almost entirely dependent on the secretion of bile acids. Thus, in this section, we will consider how bile acids are synthesized, and subsequent modifications to their structure that promote their role as biological detergents.
Bile secretion in the liver is driven primarily by the active, ATP-dependent efflux of conjugated bile acids out of the hepatocyte into the canaliculus. In some animals, there is also a variable component of bile acid-independent bile flow, although the solutes that drive this secretion are not fully understood. But in humans, canalicular bile flow is almost entirely dependent on the secretion of bile acids. Thus, in this section, we will consider how bile acids are synthesized, and subsequent modifications to their structure that promote their role as biological detergents.
Bile acids are amphipathic end products of cholesterol metabolism. The term amphipathic refers to the fact that bile acids have both a hydrophobic and hydrophilic face, and form micelles. This is essential to their physiologic function, as will be discussed later.
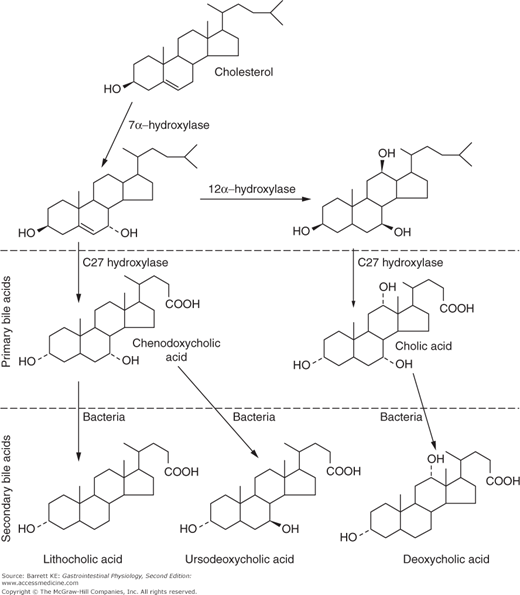
Synthesis of bile acids from cholesterol occurs in the hepatocytes, and pericentral hepatocytes are believed to be most active in this regard. Changes to both the steroid nucleus of cholesterol, as well as to its alkyl side chain, are required to convert the highly insoluble cholesterol to the water-soluble bile acid product. Bile acid synthesis in healthy humans is at a rate of approximately 200–400 mg/day. The initial, and rate-limiting, step in bile acid formation is the hydroxylation of cholesterol at the 7 position of the steroid nucleus via the enzyme cholesterol 7α hydroxylase (CYP7A1) (Figure 11–1). Note that cholesterol already contains a hydroxyl group at the 3 position, and this is retained in all of the bile acids. However, the 3-hydroxy group in cholesterol is in the β-orientation, and this is converted to the α-position by a process known as epimerization. After these initial reactions, downstream pathways diverge to yield the two primary bile acids of humans. Thus, 7α hydroxycholesterol can be acted upon by a C27 hydroxylase and several other peroxisomal enzymes, which shorten the alkyl side chain and add a carboxylic acid function to yield the bile acid chenodeoxycholic acid. In the alternate pathway, activity of the C27 hydroxylase is preceded by that of 12α hydroxylase (CYP8B1), which adds a third hydroxyl group to the steroid nucleus, ultimately yielding the tri-hydroxy bile acid, cholic acid. Changes in the expression of CYP8B1, while not rate-limiting for bile formation, result in changes in the ratio of cholic to chenodeoxycholic acid, and may thereby alter the functional properties of the resulting bile. Note that all of the hydroxyl groups in the mature bile acids are in the form of α-epimers, and are thus oriented to the same face of the molecule. Cholesterol is a flat molecule, insoluble and a major membrane constituent. In contrast, bile acids are kinked molecules that are highly water soluble when ionized.
 In humans, the only bile acids generated directly from cholesterol by endogenous enzymes are the primary bile acids, chenodeoxycholic acid and cholic acid. However, when these bile acids enter the distal small intestine or the colon, they can be acted on by bacterial enzymes to yield secondary bile acids. The most important conversion is dehydroxylation of the 7 position of the steroid nucleus, to yield lithocholic acid from chenodeoxycholic acid, and deoxycholic acid from cholic acid. Trace amounts of a third secondary bile acid, ursodeoxycholic acid (so called because it is a prominent bile acid in bears), are also generated in humans by epimerization of the 7α hydroxyl group. Although only very little ursodeoxycholic acid is formed in humans, it is important to know about this compound because it is used therapeutically. The secondary bile acids are less water soluble than the primary bile acids. Lithocholic acid, in particular, is cytotoxic if present at high concentrations, and physiologic mechanisms have developed to limit its toxicity, particularly in the bile ductules, as will be described in more detail later.
In humans, the only bile acids generated directly from cholesterol by endogenous enzymes are the primary bile acids, chenodeoxycholic acid and cholic acid. However, when these bile acids enter the distal small intestine or the colon, they can be acted on by bacterial enzymes to yield secondary bile acids. The most important conversion is dehydroxylation of the 7 position of the steroid nucleus, to yield lithocholic acid from chenodeoxycholic acid, and deoxycholic acid from cholic acid. Trace amounts of a third secondary bile acid, ursodeoxycholic acid (so called because it is a prominent bile acid in bears), are also generated in humans by epimerization of the 7α hydroxyl group. Although only very little ursodeoxycholic acid is formed in humans, it is important to know about this compound because it is used therapeutically. The secondary bile acids are less water soluble than the primary bile acids. Lithocholic acid, in particular, is cytotoxic if present at high concentrations, and physiologic mechanisms have developed to limit its toxicity, particularly in the bile ductules, as will be described in more detail later.
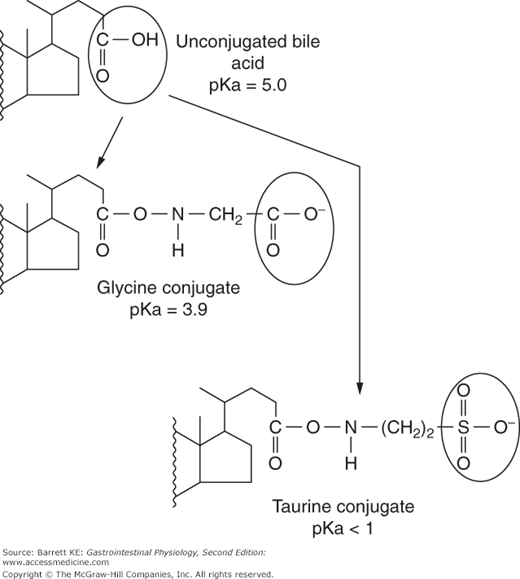
Following hepatic synthesis, both chenodeoxycholic acid and cholic acid are then further modified in the hepatocyte by conjugating them to the amino group of either glycine or taurine in a stable amide linkage (Figure 11–2). Likewise, secondary bile acids that return to the liver are also conjugated with glycine or taurine in the hepatocyte. It is these conjugated bile acids that are the substrates for active transport across the canalicular membrane. Conjugation also renders the bile acids more water-soluble, and alters their physicochemical properties as will be discussed further later.
In addition to bacterial conversion of primary to secondary bile acids, bacteria can deconjugate both primary and secondary bile acids, making them more lipophilic. Unconjugated bile acids can be considered “damaged” and can be passively absorbed across the wall of the intestine. They then travel through the portal vein back to the liver, where they are reconjugated in the hepatocyte. Thus, all bile acids secreted by the hepatocyte are in their conjugated forms.
Special handling applies to the potentially toxic bile acid, lithocholic acid. In addition to conjugation with glycine or taurine, lithocholic acid is sulfated, particularly if present in abnormally high concentrations. This increases the hydrophilicity of the molecule and is therefore believed to reduce its cytotoxic effects, which might otherwise lead to liver injury, especially if bile flow is reduced. The sulfated conjugates of lithocholic acid also cannot be absorbed by the intestine, which results in their elimination from the pool of bile acids that circulate enterohepatically (of which more will be learned later).
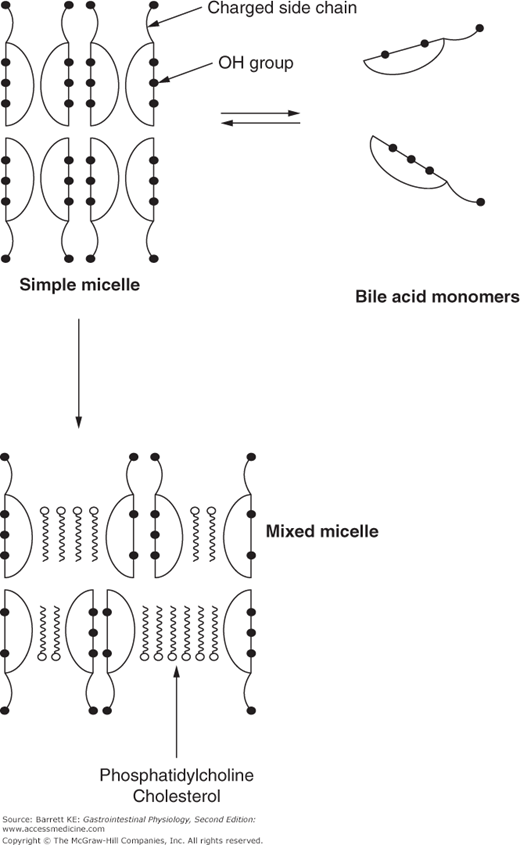
The reactions involved in bile acid biosynthesis yield amphipathic molecules with both hydrophobic and hydrophilic faces. This characteristic is vital to the physiologic function of these molecules. Although they have an appreciable solubility in water as monomers, above a certain concentration (called the critical micellar concentration, or CMC), bile acid molecules spontaneously self-associate into structures known as micelles, in which the hydrophobic faces are masked from the surrounding aqueous environment (Figure 11–3). However, simple micelles composed of bile acids alone do not exist in bile or intestinal content. In bile, bile acids form mixed micelles with phosphatidylcholine. In turn, these mixed micelles can serve as the “solvent” for hydrophobic molecules such as cholesterol or products of lipid digestion.
As noted earlier, conjugation also alters the physicochemical properties of bile acids. Most importantly, the process of conjugation decreases the pKa of bile acids from approximately 5 to 4 for glycine conjugates, or less than 2 for taurine conjugates (Figure 11–2). As a consequence, at physiologic pH, conjugated bile acids are fully ionized and are present in both bile and intestinal contents as anions. Due to this charge, conjugated bile acids are incapable of crossing cell membranes by passive means, needing instead an active transport mechanism for their secretion or uptake (Figure 11–4). This becomes important in allowing for appropriate enterohepatic circulation of bile acids in a manner coordinated with the period when they are needed to help digest the meal.
Bile Composition
Bile undergoes various alterations in its composition as it moves through the biliary system. These changes reflect both active and passive transport events and the activity of specific enzymes.
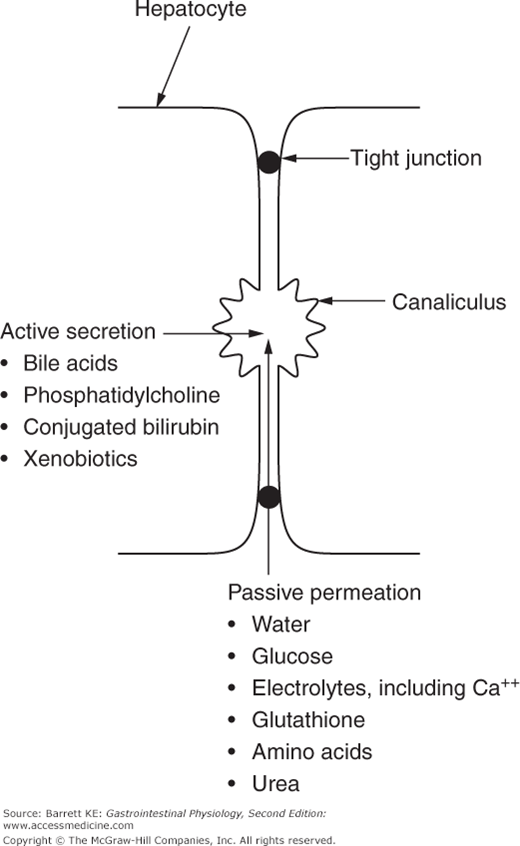
 The secretion of bile begins when bile acids are actively secreted across the canalicular membrane. Because the bile acids constitute osmotically active particles, canalicular bile is transiently hyperosmotic. However, the tight junctions that delineate the canaliculus are relatively permeable, and so water is drawn into the canaliculus to balance this, along with plasma cations to maintain electrical neutrality. Of these cations, calcium, which is present at concentrations that approximate those in plasma (1 mM), is particularly important from a pathophysiologic standpoint because it can form insoluble precipitates with certain bile solutes, such as unconjugated bilirubin, under adverse conditions. Other secondary solutes also enter bile passively from the plasma, including glutathione, glucose, amino acids, and urea.
The secretion of bile begins when bile acids are actively secreted across the canalicular membrane. Because the bile acids constitute osmotically active particles, canalicular bile is transiently hyperosmotic. However, the tight junctions that delineate the canaliculus are relatively permeable, and so water is drawn into the canaliculus to balance this, along with plasma cations to maintain electrical neutrality. Of these cations, calcium, which is present at concentrations that approximate those in plasma (1 mM), is particularly important from a pathophysiologic standpoint because it can form insoluble precipitates with certain bile solutes, such as unconjugated bilirubin, under adverse conditions. Other secondary solutes also enter bile passively from the plasma, including glutathione, glucose, amino acids, and urea.