Fig. 1
Keratinocytes, epidermal immune cells, and sensory neuron afferents communicate via cell–cell signaling factors. Keratinocytes release factors that influence immune cell activity (A), such as chemokines and cytokines. Immune cells also release chemokines and cytokines (A) that modulate keratinocyte activity. Both keratinocytes (B) and immune cells (C) can also release pruritogens, algogens, and antinociceptive factors that all act on sensory neurons, whereas sensory neurons release neuropeptides that can target both keratinocytes (B) and immune cells (C)
2 The Skin and the Detection of Itch Stimuli
The skin is a stratified epithelium, home to a variety of epithelial cells, resident immune cells, and innervating somatosensory nerve fibers (Fig. 2). Keratinocytes, the resident epithelial cells making up the epidermis, adopt unique morphologies and differentiation states as they progress from deeper to more superficial layers. Those attached to the basement membrane in the stratum basale remain proliferative, while daughter cells in the stratum spinosum exit the cell cycle. Tight junctions connect the flattened keratinocytes in the stratum granulosum, forming a cornified envelope. Finally, in the outer layer, the stratum corneum, squamous keratinocytes are continually shed as new cells proliferate and replace them from below (Simpson et al. 2011). The stratum corneum is known to play an important role in the prevention of itch disorders such as atopic dermatitis (Hatano et al. 2009).
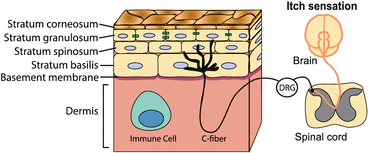

Fig. 2
The skin is a layered epithelium containing sensory nerves and immune cells. The epithelium is made up of several layers of differentiated keratinocytes. In the stratum granulosum, tight junctions (green) connect neighboring keratinocytes. Unmyelinated, nociceptive C-fibers detect pain, temperature, and itch. The cell bodies of primary sensory fibers are in the dorsal root ganglia (DRG) and project to the spinal cord. Interactions between keratinocytes, sensory neurons, and resident immune cells play an important role in chronic itch
The skin-resident immune population includes macrophages, dendritic cells, mast cells, and γδ T cells. Macrophages in the skin function much as they do in the body, by orchestrating inflammatory responses and clearing invading pathogens, cellular debris, and apoptotic cells through phagocytosis. Dendritic cells may reside in the epidermis as Langerhans cells or the dermis as dermal dendritic cells (Tay et al. 2013). Both dermal dendritic and Langerhans cells function primarily to recognize and capture antigens present in the skin for processing and presentation in initiation of the adaptive immune response. Mast cells are particularly abundant in tissues that form interfaces with the external environment, like the skin, suggestive of their role as the first line of host defense. Upon activation, mast cells release potent inflammatory mediators that are stored in their secretory vesicles, such as histamine, proteases, cytokines, proteoglycans, and lipid mediators. γδ T cells are a subset of T cells that are highly enriched in the epithelium. They have been proposed to play roles in monitoring and destroying stressed epithelial cells, promoting wound healing, and regulating inflammatory responses (Girardi 2006).
Somatosensory neuron terminals densely innervate the skin. Itch and other environmental stimuli, such as mechanical force and temperature, are detected by the somatosensory system. The cell bodies of somatosensory neurons are located in the trigeminal ganglia near the base of the skull and dorsal root ganglia (DRG) adjacent to the spinal cord. Sensory neurons in the trigeminal ganglia innervate the skin and organs of the head and face and send efferent projections directly to the brain stem. Sensory neurons in the DRG project peripheral afferent fibers to the skin and organs in the rest of the body and project central fibers to the dorsal horn of the spinal cord, where they synapse onto dorsal horn neurons that ultimately send signals to the brain stem and thalamus (Fig. 1).
A heterogeneous population of primary sensory neurons mediate itch and terminate in the skin as free nerve endings (Dong et al. 2001; Imamachi et al. 2009; Han et al. 2013; Bautista et al. 2014). Approximately 5–20 % of the total population of primary afferent C-fibers are activated by endogenous itch-producing compounds released by nonneuronal cells in the skin (e.g., mast cell-derived histamine), as well as by exogenous pruritogens, such as chloroquine (Ikoma et al. 2006; Imamachi et al. 2009; Liu et al. 2009). Pruriceptors are categorized by the compounds they respond to (e.g., histamine, serotonin, and chloroquine) and the compounds they release (e.g., neuropeptides like calcitonin gene-related polypeptide (CGRP) or neurotransmitters like glutamate). CGRP-expressing neurons have been shown to be required for full expression of chloroquine- and histamine-evoked itch behaviors (McCoy et al. 2013) and terminate in the stratum spinosum (Lumpkin and Caterina 2007). Interestingly, different layers of the epidermis secrete molecules with opposing roles in pain and itch. In the superficial epidermis, stimulation of keratinocytes results in the release of the antinociceptive molecule beta-endorphin, while deeper keratinocytes release pro-nociceptive endothelin-1 (ET-1) when stimulated (Lumpkin and Caterina 2007). These data suggest that the different epidermal layers may specialize in specific somatosensory modalities, though how these layer-specific differences pertain to the sensation of itch remains unknown.
3 Keratinocyte-Mediated Modulation of Pruriceptors
Keratinocytes interact with neurons to promote itch both indirectly and directly. First, keratinocytes can promote itch indirectly through the release of inflammatory mediators that activate immune cells, which in turn stimulate pruriceptors. Second, keratinocytes are the primary cellular component of the epidermis. Physical disruption of the skin barrier enables entry of exogenous pruritogens into the body, indirectly promoting itch and inflammation. Direct interactions between keratinocytes and neurons occur via the release of pruritogens by activated keratinocytes, as well as the release of growth factors that encourage nerve sprouting and sensitization. Both direct and indirect interactions between neurons and keratinocytes are facilitated by the location of itch-transducing C-fibers as free nerve endings in the skin. Peptidergic itch-transducing C-fibers terminate in the stratum spinosum, while non-peptidergic C-fibers penetrate more superficially to the stratum granulosum (Zylka et al. 2005). Although classical synapses have not been observed between keratinocytes and neurons, electron microscopy of the skin reveals membrane–membrane apposition, where gap junctions or chemical synapses could exist (Hilliges et al. 1995).
4 Indirect Interactions Between Keratinocytes and Sensory Neurons
Upon activation by pruritogens, keratinocytes release a number of inflammatory molecules, which have been proposed to account for enhanced pruriceptor sensitivity under chronic itch conditions. For example, histamine can act on keratinocytes to increase the production of proinflammatory cytokines such as IL-6 and IL-8 (Kohda et al. 2002) and the proinflammatory chemokine CCL5 (Giustizieri et al. 2004). Similarly, keratinocytes treated with substance P or CGRP upregulate the expression of the inflammatory cytokine IL-1α and inflammatory chemokine IL-8 at both the mRNA and protein levels (Dallos et al. 2006). Keratinocytes can also be activated by PAR agonists (e.g., tryptase), endothelin-1 (ET-1), vasoactive intestinal polypeptide (VIP), and galanin (Yohn et al. 1994; Santulli et al. 1995; Dallos et al. 2006). Some chemokines and cytokines released by keratinocytes activate sensory neurons directly, while others recruit immune cell populations such as mast cells and T cells, which can then release sensory neuron-activating pruritogens like histamine and IL-31, respectively (Fig. 3; Tay et al. 2013). IL-31 can directly activate neurons in culture, and injection of IL-31 causes robust scratching that is independent of mast cells (Cevikbas et al. 2014). The dysregulation of these feedback loops among keratinocytes, immune cells, and sensory neuron afferents is common in chronic itch diseases of the skin such as psoriasis (Raychaudhuri and Raychaudhuri 2004; Raap et al. 2011).
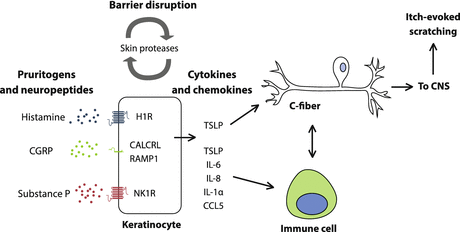

Fig. 3
Keratinocytes indirectly promote itch in sensory nerves. Keratinocytes are activated by pruritogens and neuropeptides such as histamine, CGRP, and SP. Keratinocytes release chemokines and cytokines that activate the immune system and result in release of pruritogens like TSLP and IL-31. TSLP also activates sensory neurons, inducing itch directly. Sensory neurons and immune cells also interact, for example, through the release of IL-31 by T cells and inflammatory mediators by neurons. In addition, chronic itch is associated with disruption of barrier function, which is maintained by keratinocytes. Barrier disruption may result from increased levels of skin proteases, such as kallikreins and tryptase, which normally regulate stratum corneum turnover
Keratinocytes are also critical for maintaining the epidermal barrier, which prevents both entry of exogenous objects and exit of internal components. Thus, the epidermal barrier disruption common to chronic itch disorders may facilitate entry of pruritogens and allergens. For example, in the NC/Nga atopic dermatitis (AD) mouse model, scratching itself exacerbates the itch phenotype (Hirano et al. 2011). Indeed, in AD patients, lesions develop at the site of scratching, and barrier defects in the skin are frequently associated with the development of AD (Elias and Schmuth 2009; Leung 2013). Epidermal serine proteases known as kallikreins (KLK) also mediate a positive feedback loop between keratinocyte activation and barrier disruption. KLKs function at the outer layer of the epidermis, enabling desquamation of the stratum corneum. Activated keratinocytes release KLKs, which in turn break down the corneodesmosomes holding together the outer layer of keratinocytes, thereby enabling shedding of the top layer of the epidermis and its replacement by new cells from the stratum granulosum (Tanaka et al. 2011). Unregulated KLK activity results in excessive loss of outer skin cells, and improper KLK regulation is believed to contribute to the impaired skin barrier seen in AD (Tanaka et al. 2011). As such, transgenic mice with overexpression of KLK5 in the stratum corneum and stratum granulosum have defective skin barrier function and significantly higher transepidermal water loss than wild-type mice. Further, these mice exhibit continuous scratching (Furio et al. 2014).
Physical perturbation of keratinocytes may also lead more directly to itch, as tape stripping results in increased production of the cytokine thymic stromal lymphopoietin (TSLP) (Kabashima 2013), which can activate itch directly through sensory neurons as well as indirectly via immune cells (Ziegler and Artis 2010; Wilson et al. 2013b). KLK5, in addition to physically disrupting the epidermis, also activates PAR2 in keratinocytes, leading to expression of several inflammatory mediators including TSLP (Stefansson et al. 2008; Briot et al. 2009). Another protease that leads to itch is cathepsin S, a cysteine protease that also activates PAR2 (Reddy et al. 2010; Elmariah et al. 2014). Further, overexpression of cathepsin S leads to atopic dermatitis phenotypes in mice, and levels correlate with seborrhoeic dermatitis in humans (Kim et al. 2011; Viodé et al. 2014).
5 Direct Interactions Between Keratinocytes and Sensory Neurons
Keratinocytes release a number of pruritogens that can directly activate itch fibers, including histamine, TSLP, and ET-1 (Fig. 4; Lumpkin and Caterina 2007). Histamine is a pruritogen that is secreted by a variety of cells including mast cells (Chatterjea and Martinov 2014), basophils, and keratinocytes. Cultured keratinocytes release histamine upon radiation by ultraviolet light B (Malaviya et al. 1996), and the release of histamine by keratinocytes was recently confirmed in vivo (Inami et al. 2013). Histamine acts directly on primary sensory neurons to induce itch primarily through the H1 receptor (Jeffry et al. 2011). The ion channel TRPV1 is required for both histamine-evoked calcium signals in DRG neurons and histamine-evoked itch behaviors in mice (Kim et al. 2004; Imamachi et al. 2009).
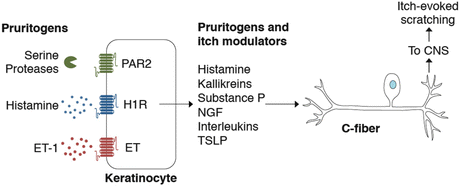

Fig. 4
Keratinocytes act in concert with somatosensory neurons to detect and transduce itch stimuli. Keratinocytes are activated by a number of pruritogens including histamine, SLIGRL, and ET-1 and secrete pruritogens and other itch mediators in response. C-fibers terminate as free nerve endings in the skin, enabling rapid signaling between keratinocytes and itch fibers
As mentioned above, TSLP can also be released by keratinocytes upon PAR2 activation. Cleavage of the N terminus of the PAR2 receptor leads to the release of intracellular calcium stores, causing activation of STIM1 and influx of extracellular calcium through the channel ORAI1. Calcium influx in turn activates calcineurin, which dephosphorylates NFAT and enables its translocation to the nucleus. NFAT is a transcriptional activator of TSLP, leading to release of TSLP into the extracellular space (Fig. 5; Wilson et al. 2013b). TSLP directly activates sensory neurons through the TSLP receptor and requires the ion channel TRPA1 (Wilson et al. 2011, 2013a, b). TSLP release by keratinocytes also contributes indirectly to itch. TSLP promotes inflammatory immune responses, leading to increased cytokine production by mast cells and T helper type 2 (TH2) differentiation of CD4+ T cells (Ziegler and Artis 2010). However, while activation of immune responses by TSLP plays a key role in allergic inflammation and contributes to the development of chronic itch, neither T and B cells nor mast cells are required for acute TSLP-induced itch (Wilson et al. 2013b).
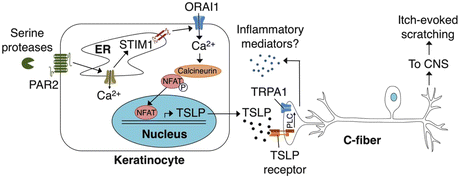

Fig. 5
Schematic diagram depicting the ORAI1 signaling pathway in keratinocytes that links PAR2 to TSLP secretion and activation of itch neurons. Activation of PAR2 triggers release of Ca2+ from the ER and activation of STIM1, which opens ORAI1 channels to promote Ca2+ influx. Ca2+ activates the phosphatase calcineurin, which dephosphorylates NFAT and causes nuclear translocation, thus inducing transcription of TSLP. Secreted TSLP depolarizes a subset of C-fibers to evoke itch, in a TSLPR- and TRPA1-dependent manner. Activation of TRPA1-expressing sensory neurons can then lead to release of neuropeptides in the skin in a process known as neurogenic inflammation
Keratinocytes, along with sensory neurons and immune cells, also secrete ET-1, a pruritogen that directly activates ~3 % of DRG neurons in culture (Kido-Nakahara et al. 2014). Injection of ET-1 has been reported to induce either pain or itch or both pain and itch in mice (Khodorova et al. 2003; Liang et al. 2010; Gomes et al. 2012; Kido-Nakahara et al. 2014). However, when ET-1 is injected intradermally into the mouse cheek, an assay used to distinguish itch from pain, both wiping (indicative of pain) and scratching (indicative of itch) occur (Gomes et al. 2012). To further complicate the action of ET-1, two ET-1 receptors, ETA and ETB, are expressed in the skin and exert opposing effects. ETA is expressed by neurons and mast cells and activation promotes scratching. In contrast, ETB is expressed by keratinocytes and appears to inhibit both itch and pain (Gomes et al. 2012). Scratching responses to ET-1 are dependent on the ion channel TRPA1 but not TRPV1. However, loss of TRPA1 does not affect the proportion of DRG neurons that respond to ET-1 in culture (Kido-Nakahara et al. 2014). Further studies are needed to understand the exact roles of ET-1 receptors in somatosensation.
Keratinocytes also mediate itch by the release of the neurotrophin nerve growth factor (NGF). When treated with substance P or CGRP, keratinocytes increase expression of NGF, which sensitizes somatosensory neurons and may contribute to increased innervation in chronic itch conditions (Urashima and Mihara 1998; Dallos et al. 2006). NGF secreted by keratinocytes is able to induce neurite outgrowth in culture (Di Marco et al. 1991), and NGF also interacts with the immune system to degranulate mast cells and activate T lymphocytes (Raychaudhuri and Raychaudhuri 2004).
6 Neuronal Modulation of Keratinocytes
Neurons, in turn, influence keratinocytes by releasing inflammatory mediators that can activate keratinocytes, as well as by promoting epidermal thickening and the proliferation of keratinocytes. As mentioned above, keratinocytes are activated by a number of pruritogens and inflammatory mediators, including histamine, substance P, and CGRP.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


