Compound
CAS number
Molecular formula
Chemical structure
Synonyms
Bis-(2-chloroethyl) sulfide
505-60-2
C4-H8-Cl2-S
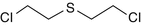
HD, agent HD, mustard gas, yperite, LOST, Sulfur mustard
1,2-Bis-(2-chloroethylthio)-ethane
3563-36-8
C6-H12-Cl2-S2
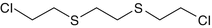
Q, agent Q, sesquimustard, 1,8-dichloro-3,6-dithiaoctane
Bis-(2-chloroethylthioethyl)-ether
63918-89-8
C8-H16-Cl2-O-S2
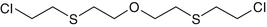
T, agent T, O-mustard, 2-2′-Di(3-chloroethylthio)-diethyl ether
2-Chloroethyl chloromethyl sulfide
2625-76-5
C3-H6-S-Cl2
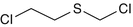
Ethane, 1-chloro-2-[(chloromethyl)thio]-
Bis-(2-chloroethylthio)-methane
63869-13-6
C5-H10-Cl2-S2

HK, Bis(2-chloroethylthiomethyl)ether
Bis-1,3-(2-chloroethylthio)-n-propane
63905-10-2
C7-H14-Cl2-S2

1,9-Dichloro-3,7-dithianonane
Bis-1,4-(2-chloroethylthio)-n-butane
142868-93-7
C8-H16-Cl2-S2

1,4-Bis (2-chloroethylthio) butane
Bis-1,5-(2-chloroethylthio)-n-pentane
142868-94-8
C9-H18-Cl2-S2

Pentane, 1,5-bis((2-chloroethyl)thio)-
Bis-(2-chloroethylthiomethyl)-ether
63918-90-1
C6-H12-Cl2-o-S2
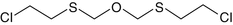
Ethane,1,1′(oxybis(methylenethio))bis(2-chloro-
Bis-(2-chloroethyl)ethylamine
538-07-8
C6H13Cl2N
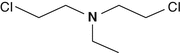
HN-1, Nitrogen mustard (HN-1), Ethylbis(2-chloroethyl)amine
Bis-(2-chloroethyl)methylamine
51-75-2
C5H11Cl2N

HN-2, Nitrogen mustard (HN-2), Mechlorethamine, Chlormethine, Mustine
Tris-(2-chloroethyl)amine
555-77-1
C6H12Cl3N
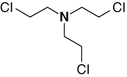
HN-3, Nitrogen mustard (HN-3), Trichlormethine, Trimustine, 2,2′,2″-Trichlorotriethylamine
Table 3.2
Physico-chemical properties of mustard compounds (www.chem.sis.nlm.nih.gov/chemidplus)
Physical properties | SM | HN-1 | HN-2 | HN-3 |
|---|---|---|---|---|
Melting point (°C) | 13.5 | −3.40E + 01 | −6.00E + 01 | −4.00E + 00 |
Boiling point (°C) | 216 | 194 | 87 | |
log P (octonal- water) | 2.410 | 2.02 | 0.91 | 2.270 |
Water solubility (mg/L) | 684 | 160 | 1.20E + 04 | 1600 |
Vapor pressure (mm Hg) | 0.11 | 0.25 | 65.1 | 0.011 |
Henry’s law constant (atm-m3/mole) | 3.37E-0.5 | 3.36E-04 | 8.48E-08 | 1.85E-06 |
Atmospheric OH rate constant (cm3/molecule-s) | 7.82E-12 | 1.59E-11 | 8.39E-12 | 1.07E-11 |
3.2 Pharmaco- and Toxico- Kinetics
3.2.1 Sulfur Mustard
3.2.1.1 Absorption
Skin, eyes, and respiratory tract are the main routes of exposure to mustards whose oily nature causes them to persist longer on the exposed surface of the body, giving more time for exerting local effects or systemic absorption. High lipid solubility of mustards, further, facilitates their passage through cell membrane leading to the high rate of absorption (ATSDR 2003). Mustard compounds can be absorbed through the skin, eyes, respiratory and GI tract. Both vapor and liquid forms of the SM can penetrate through the skin with a rate estimated to be 1–4 μg/cm2/min at 25 °C. However, the rate of dermal penetration of SM is dependent on the dose, temperature, humidity, and thickness of the skin. The base of the hair shaft or hair follicle, which have a thinner epithelial tissue are suitable for more absorption of SM applied cutaneous (Young and Bast 2009). It has been estimated that around 80 % of total dose applied to the skin evaporates while 10 % absorbs systemically and 10 % remains at the site of exposure that is responsible for local effects. The rate of dermal absorption can reach up to 90 % when applied by occlusion with an exposure duration of 6 h (Hambrook et al. 1993). When administered by inhalation, SM is absorbed 70–90 % through the mucous membrane of the nasal system (Papirmeister et al. 1984).
3.2.1.2 Distribution
Upon entering into the blood, SM highly tends to bind hemoglobin and then glutathione (Hambrook et al. 1993). Because of high lipid solubility, SM and its metabolites can be widely distributed in the body after intravenous or percutaneous exposure. Regardless of the route of exposure, the equilibrium between blood and tissue levels of SM is established 5 min after systemic absorption. Maximum levels can be detected in the lung, liver, and kidney, however, postmortem and in vivo studies has indicated that SM can be efficiently distributed to the other organs including fat, brain, muscle, spleen, adrenals, bone marrow, cerebral fluid, and abdominal skin. Within 5 min after ocular application, SM is shown to be concentrated in the cornea, but lesser extent can also be found in the iris, lens, and conjunctiva (Axelrod and Hamilton 1947).
3.2.1.3 Metabolism
In aqueous conditions, SM undergoes intramolecular cyclization producing ethylene episulfonium ion which is a hyperactive compound and tends to react with electron rich molecules such as –SH and –NH2. The main metabolic pathway is hydrolysis by which SM is converted to thiodiglycol and then s-oxidation create sulfoxide and sufone. These products are finally conjugated and excreted mainly in the urine. The major urinary metabolites include glutathione-bis-chloroethyl sulfide conjugates (45 %), thiodiglycol plus its conjugates (14.4 %), sulfone conjugates (7 %) and minute amounts of cysteine-bis-(β-chloroethyl) sulfone which is produced under the effect of β-lyase on cysteine. It has been estimated that urinary concentration of thiodiglycol reaches to the peak on post-exposure day 4 and can be detected in the urine up to 2 weeks. It has the first-order elimination kinetics with a half-life 1.2 days. Since active metabolites of SM are capable to react with nucleophiles found in the structure of DNA and glutathione, some DNA adducts can also be detected in the urine like N7-(2-hydroxyethylthioethyl)-2ʹ-deoxyguanosine and 2ʹ-deoxyguanosine derivatives of N7-HETE-guanine (TOXNET 2013).
3.2.1.4 Elimination
The main route of elimination of SM is urinary with the first order pattern and its metabolites sometimes detected in the urine for up to 3 months. In an experiment on the rat, urine and feces half-lives of SM were estimated 1.4 and 1.6 days, respectively (TOXNET 2013).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


