CHAPTER 22 Basal ganglia
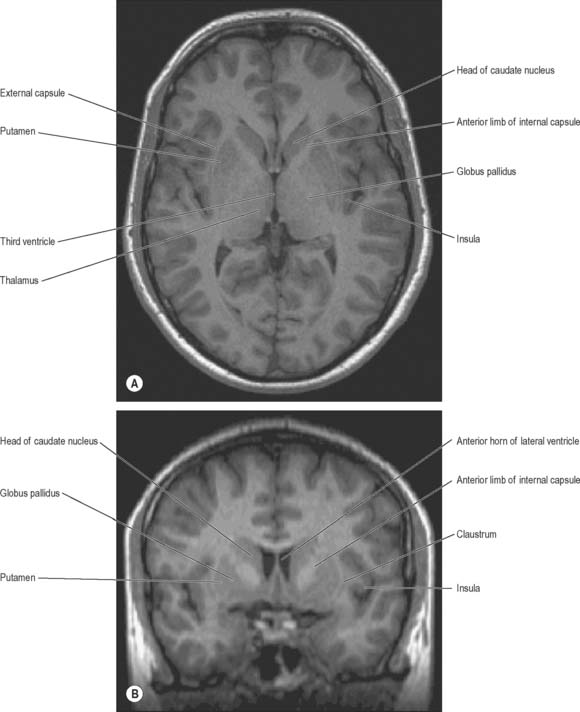
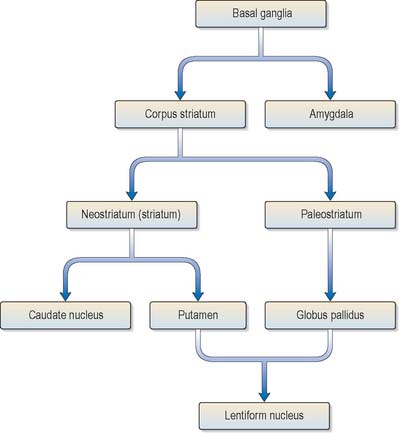
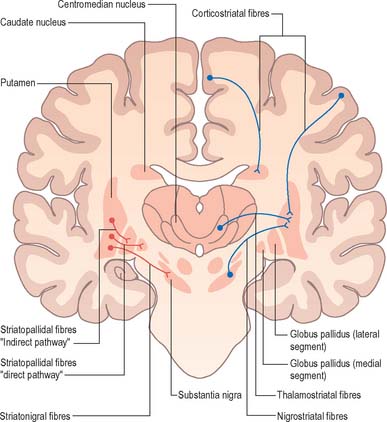
The term basal ganglia is used to denote a number of subcortical nuclear masses that lie in the inferior part of the cerebral hemisphere, in close relation with the internal capsule (Figs 22.1, 22.2; see Fig. 23.28). The traditional definition of the basal ganglia included the corpus striatum, claustrum, and amygdaloid complex. The term has now been restricted to the corpus striatum and its associated structures in the diencephalon and midbrain; collectively they form a functional complex involved in the control of movement and motivational aspects of behaviour. The function of the claustrum is unknown; the amygdala is more closely related to the limbic system and is therefore described in that context (see Ch. 23).
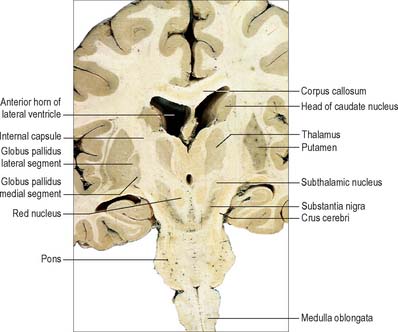
Fig. 22.2 Oblique coronal section of the brain.
(Dissection by EL Rees; photograph by Kevin Fitzpatrick on behalf of GKT School of Medicine, London.)
The corpus striatum consists of the caudate nucleus, putamen and globus pallidus (Fig. 22.3). Because of their close proximity, the putamen and globus pallidus were once considered as an entity, termed the lentiform (lenticular) complex or nucleus. However, although the name has been retained in gross anatomical terminology and in some compound names (e.g. sublenticular, retrolenticular), it is now known that the putamen and caudate nucleus share a common chemocytoarchitecture and connections, and they are referred to jointly as the neostriatum or simply the striatum.
Disorders of the basal ganglia are principally characterized by abnormalities of movement, muscle tone and posture. There is a wide spectrum of clinical presentations ranging from poverty of movement and hypertonia at one extreme (typified by Parkinson’s disease) to abnormal involuntary movements (dyskinesias) at the other. The underlying pathophysiological mechanisms that mediate these disorders have been much studied in recent years and are better understood than for any other type of complex neurological dysfunction. This has led to the introduction of new rational therapeutic strategies for both medical and neurosurgical treatment of movement disorders.
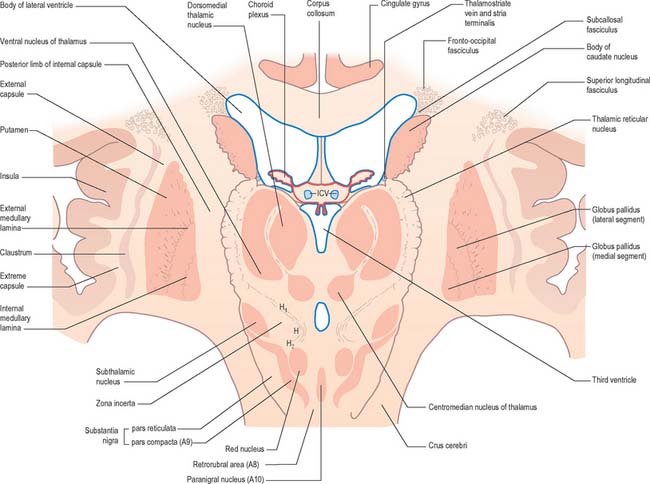
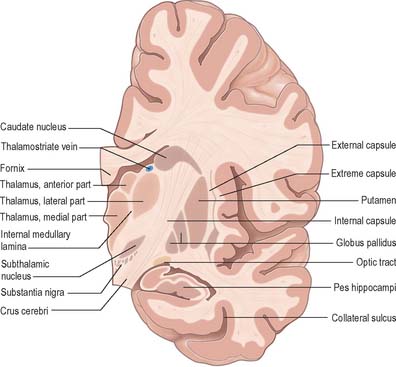
The caudate nucleus is a curved, tadpole-shaped mass. It has a large anterior head, which tapers to a body, and a down-curving tail (Fig. 22.4). The head is covered with ependyma and lies in the floor and lateral wall of the anterior horn of the lateral ventricle, in front of the interventricular foramen. The tapering body is in the floor of the body of the ventricle, and the narrow tail follows the curve of the inferior horn, and so lies in the ventricular roof, in the temporal lobe. Medially, the greater part of the caudate nucleus abuts the thalamus, along a junction that is marked by a groove, the sulcus terminalis. The sulcus contains the stria terminalis, lying deep to the ependyma (Fig. 22.5). The sulcus terminalis is especially prominent anterosuperiorly (because of the large size of the head and body of the caudate nucleus relative to the tail), and here the stria terminalis is accompanied by the thalamostriate vein.
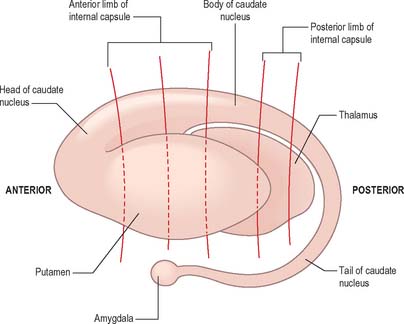
Fig. 22.4 The striatum within the left cerebral hemisphere.
(By permission from Crossman AR, Neary D 2000 Neuroanatomy, 3rd edn. Edinburgh: Churchill Livingstone.)
The corpus callosum lies above the head and body of the caudate nucleus. The two are separated laterally by the fronto-occipital fasciculus, and medially by the subcallosal fasciculus which caps the nucleus (Figs 22.5, 22.6). The caudate nucleus is largely separated from the lentiform complex by the anterior limb of the internal capsule (Figs 22.1, 22.6 and 22.7). However, the inferior part of the head of the caudate becomes continuous with the most inferior part of the putamen immediately above the anterior perforated substance; this junctional region is sometimes known as the fundus striati (Fig. 22.6). Variable bridges of cells connect the putamen to the caudate nucleus for most of its length. They are most prominent anteriorly, in the region of the fundus striati and the head and body of the caudate nucleus, where they break up the anterior limb of the internal capsule (Fig. 22.7). In the temporal lobe, the anterior part of the tail of the caudate nucleus becomes continuous with the posteroinferior part of the putamen. The vast bulk of the caudate nucleus and putamen are often referred to as the dorsal striatum. A smaller inferomedial part of the rostral striatum is referred to as the ventral striatum, and includes the nucleus accumbens.
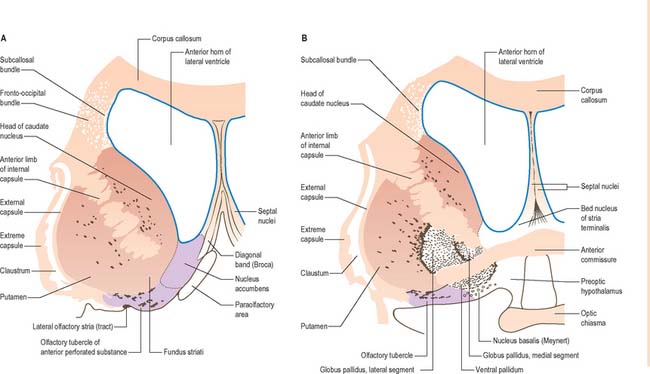
Fig. 22.6 Coronal sections through the corpus striatum and anterior perforated substance. A is anterior to B.
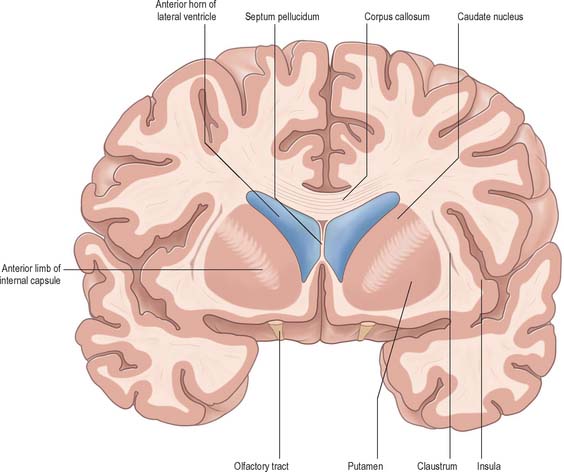
Fig. 22.7 Posterior aspect of a coronal section through the anterior horn of the lateral ventricles.
The lentiform complex (Figs 22.1, 22.2, 22.8) lies deep to the insular cortex, with which it is roughly coextensive, although they are separated by a thin layer of white matter and by the claustrum. The latter splits the insular subcortical white matter to create the extreme and external capsules; the external capsule separates the claustrum from the putamen. The internal capsule separates the lentiform complex from the caudate nucleus.
Inferiorly, a little behind the fundus striati, the lentiform complex is grooved by the anterior commissure, which connects inferior parts of the temporal lobes and the anterior olfactory cortex of the two sides (Fig. 22.6). The area above the commissure is referred to as the dorsal pallidum, and that below it as the ventral pallidum.
STRIATUM
The striatum consists of the caudate nucleus, putamen and ventral striatum, which are all highly cellular and well vascularized. The caudate and putamen are traversed by numerous small bundles of thinly-myelinated, or non-myelinated, small-diameter axons, which are mostly striatal afferents and efferents. They radiate through the striatal tissue as though converging on, or radiating from, the globus pallidus. The bundles are occasionally referred to by the archaic term ‘Wilson’s pencils’ and they account for the striated appearance of the corpus striatum.
All afferents to the striatum terminate in a mosaic manner. The size of a cluster of terminals is usually 100–200 μm across. Some afferent terminal clusters are not arranged in register with the clear striosome/matrix distributions seen in nigrostriatal and thalamostriatal axons. In general, afferents from neocortex end in striatal matrix and those from allocortex end in striosomes. However, the distinction is not absolute: although afferents from the neocortex arise in layers V and VI, those from the superficial part of layer V end predominantly in striatal matrix, whereas those from deeper neocortex project to striosomes. Striatal cell bodies, which are the sources of efferents, also form clusters, but again are not uniformly related to striosomes. For example, the cell bodies of some striatopallidal and striatonigral axons lie clustered within striosomes, and others lie outside them, but still in clusters. The neurones and neuropil of the ventral striatum are essentially similar to those of the dorsal striatum, but the striosomal/matrix organization is less well-defined, and seems to consist predominantly of striosomes.
The major connections of the striatum are summarized in Fig. 22.9. Although the connections of the dorsal and ventral divisions overlap, the generalization can be made that the dorsal striatum is predominantly connected with motor and associative areas of the cerebral cortex, whilst the ventral striatum is connected with the limbic system and orbitofrontal and temporal cortices. For both dorsal and ventral striatum, the pallidum and substantia nigra pars reticulata are key efferent structures. The fundamental arrangement is the same for both divisions. The cerebral cortex projects to the striatum, which in turn projects to the pallidum and substantia nigra pars reticulata, and efferents from the pallidum and substantia nigra pars reticulata influence the cerebral cortex (either the supplementary motor area or prefrontal and cingulate cortices via the thalamus) and the superior colliculus (see below).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree