Autogenous Arteriovenous Hemodialysis Access
Robert J. Feezor
Thomas S. Huber
Introduction
End stage renal disease (ESRD), and the maintenance of hemodialysis access, is a tremendous public health problem that has reached near epidemic proportions in the United States. In 2006, there were 506,256 prevalent (prevalence—patient count at a single point in time) and 110, 854 incident (incidence—new patients over a time interval) ESRD patients. Notably, this represents a threefold increase from 1988 and it has been estimated that these numbers will increase another 50% by 2020 with estimated prevalent and incident patient counts of 784,613 and 150,772, respectively. The majority of patients with ESRD are on hemodialysis (hemodialysis 65%, transplant 30%, and peritoneal dialysis 5%). There was a record 18,000 kidney transplants performed in 2006, largely due to an increase in the number of deceased donors. However, the number of patients on the transplant list has continued to increase and outstripped the number of available organs. Indeed, the median time on the transplant list across the country is 681 days. The Medicare costs associated with ESRD are staggering. Although ESRD patients account for only 1.1% of the general Medicare population, dialysis-related expenditures accounted for 7.4% ($22.7 billion) of the general Medicare costs in 2006. The Medicare expenditure per ESRD patient per year is greatest for the patients on hemodialysis (hemodialysis $71,889; peritoneal $53,327; transplant $24,951). Not surprisingly, maintaining hemodialysis access is a significant burden for most health care providers. This is compounded by the limited functional patency rates for the various access options and, almost paradoxically, the improving patient survival.
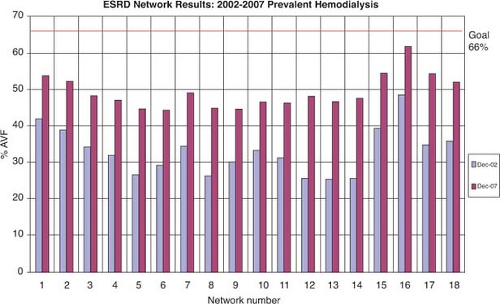
The National Kidney Foundation Dialysis Outcome Quality Initiative (KDOQI) has emphasized the importance of autogenous access. Their “preferred” access choices include the radiocephalic, brachiocephalic, and brachiobasilic autogenous access, although they concede that prosthetic or biological accesses are acceptable. It is interesting to note that there has been an evolution in the guidelines with a change in the emphasis from an “autogenous” to a “functional” access (i.e., both autogenous and prosthetic access acceptable). Not surprisingly, they recommend that long-term dialysis catheters should be avoided. The guidelines have set an autogenous access target rate of >65% with a catheter rate of <10%. The Center for Medicare and Medicaid Services (CMS) recently completed a 3-year program entitled the National Vascular Access Improvement Initiative or “Fistula First Breakthrough Initiative” (fistula—autogenous arteriovenous hemodialysis access) to achieve the KDOQI targets or specifically to “increase the proportion of hemodialysis patients who use arteriovenous fistulas (AVFs; autogenous access) as the primary mode of vascular access.” This multidisciplinary approach was defined by 11 specific concepts designed to impact practice patterns (Table 1). Notably, a significant proportion of the “Fistula First” effort was targeted at access surgeons. The “Fistula First” effort was successful in increasing the prevalence of autogenous access across the country and has increased their autogenous access target to 66% (Fig. 1). Despite these national initiatives, the prevalence of autogenous accesses in the United States remains well below the 80% rate reported from Europe (France, Germany, Italy, United Kingdom, Spain).
Table 1 Fistula First Breakthrough Initiative Practice Recommendations | |
|---|---|
|
The KDOQI recommendations for autogenous accesses are based partly upon the presumption that the patency rates for autogenous access are superior to their prosthetic counterparts. However, the data in the KDOQI cited to justify the superiority of autogenous accesses are limited by Evidence Based Medicine Standards and included retrospective case series and expert opinion. Murad et al. performed a systematic review and meta-analysis to compare autogenous and prosthetic hemodialysis access as part of the Society for Vascular Surgery Clinical Practice Guidelines and concluded that the autogenous accesses had better patency rates at both 12 and 36 months. However, they stated that these conclusions were based upon “low-quality evidence from inconsistent studies with limited protection against bias.” Our group published a formal systematic review of the literature comparing the patency rates of upper extremity polytetrafluoroethylene (PTFE) and autogenous accesses in adults. Studies were considered acceptable for
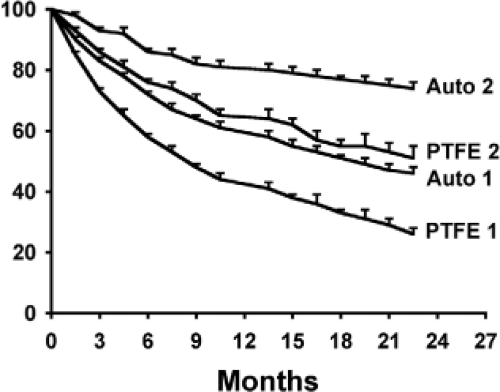
inclusion if patency was reported using either the life table or Kaplan–Meier methods and included the number of patients at risk. Unfortunately, there were only 34 studies that satisfied the inclusion criteria with the majority composed of case series or non-randomized controlled studies with the data collected in a retrospective fashion. The primary annual patency rates for the autogenous and PTFE accesses were ∼60% and 40%, respectively, whereas the corresponding secondary patency rates were 80% and 60% (Fig. 2). Notably, theses secondary patency rates are significantly lower than the outcome targets defined by KDOQI (autogenous access—thrombosis rate 0.25 episodes/patient-year at risk, patency rate >3 years; prosthetic access—thrombosis rate 0.5 episodes/patient-year at risk, patency rate >2 years).
inclusion if patency was reported using either the life table or Kaplan–Meier methods and included the number of patients at risk. Unfortunately, there were only 34 studies that satisfied the inclusion criteria with the majority composed of case series or non-randomized controlled studies with the data collected in a retrospective fashion. The primary annual patency rates for the autogenous and PTFE accesses were ∼60% and 40%, respectively, whereas the corresponding secondary patency rates were 80% and 60% (Fig. 2). Notably, theses secondary patency rates are significantly lower than the outcome targets defined by KDOQI (autogenous access—thrombosis rate 0.25 episodes/patient-year at risk, patency rate >3 years; prosthetic access—thrombosis rate 0.5 episodes/patient-year at risk, patency rate >2 years).
The KDOQI also justified the use of autogenous accesses based upon their lower complication rates. Notably, Murad et al. reported that autogenous accesses were associated with a lower infection risk in their systematic review, although there was no difference in the other complication rates. We were unable to determine accurate complication rates in our systematic review of the literature either because the complications were not described in the individual studies, the descriptions were not standardized, or the means of reporting were not amenable to meta-analysis. However, the results from the individual studies suggest that the perioperative mortality rate was essentially 0 (median 0; range 0% to 1%), whereas the incidence of hand ischemia (median 2%; range 0% to 14%), access infection (median 7%; range 0% to 30%), and aneurysm/pseudoaneurysm formation (median 4%; range 0% to 6%) were low for both autogenous and PTFE accesses. Not surprisingly, the overwhelming majority of the access infections were seen in the PTFE accesses.
The annual mortality rate associated with dialysis catheters and prosthetic accesses has been consistently shown to exceed those for autogenous accesses. Notably, Dhingra et al. reported from the United States Renal Data System that the relative mortality risks (reference group autogenous accesses) were 1.41 and 1.54 for diabetic patients dialyzed with prosthetic accesses and catheters, respectively, whereas the corresponding values were 1.08 and 1.70,
respectively, among non-diabetics. Indeed, the annual unadjusted mortality rate for all hemodialysis patients across the United States was a staggering 21.7% in the Dialysis Outcomes and Practice Patterns Study (DOPPS) and was significantly greater than those reported from Europe (15.6%) and Japan (6.6%). Although the mean patient age and the burden of comorbidities were greater in the United States, the adjusted relative risk of mortality was 1.33 greater in the United States relative to Europe and 3.78 relative to Japan. It is likely that the discrepancy in the prevalence of the autogenous accesses between the three continents contributed.
respectively, among non-diabetics. Indeed, the annual unadjusted mortality rate for all hemodialysis patients across the United States was a staggering 21.7% in the Dialysis Outcomes and Practice Patterns Study (DOPPS) and was significantly greater than those reported from Europe (15.6%) and Japan (6.6%). Although the mean patient age and the burden of comorbidities were greater in the United States, the adjusted relative risk of mortality was 1.33 greater in the United States relative to Europe and 3.78 relative to Japan. It is likely that the discrepancy in the prevalence of the autogenous accesses between the three continents contributed.
There are a few distinct disadvantages to autogenous accesses. The obligatory period necessary to allow the autogenous access to mature usually exceeds the corresponding 4- to 6-week period required for incorporation of the prosthetic accesses, although newer synthetic graft designs are touted to enable earlier cannulation. Indeed, Rayner et al. reported from the DOPPS that the median duration from autogenous access creation to cannulation in the United States was 98 days. This obligatory period usually requires the use of dialysis catheters as a “bridge” and exposes the patients to all the potential catheter-related complications. Admittedly, the use of dialysis catheters in this setting may be reduced by early patient referral to an access surgeon prior to the initiation of dialysis. Furthermore, a significant proportion of the autogenous accesses fail to mature sufficient for cannulation. Dember et al. reported from the Dialysis Access Consortium (a multicenter randomized trial evaluating the role of clopidogrel on access maturation) that the failure to mature rate for autogenous accesses was >60%. Lok et al. developed a scoring system to predict maturation and found that age, race, peripheral arterial occlusive disease, and coronary artery occlusive disease were all associated with failure. Using their system, the predicted “failure to mature” rate for an autogenous access in a 70-year-old African-American with both coronary artery disease and peripheral vascular disease was ∼70%. Last, ∼25% of the initial autogenous accesses need some type of remedial imaging or procedure to facilitate maturation. This prolongs the requisite time that an individual patient requires a catheter.
Despite these limitations, it is generally well accepted that a “mature” autogenous accesses is the optimal choice for permanent hemodialysis access, as recommended by the national initiatives. We have attempted to optimize the use of autogenous access in our own practice, but would concede that an autogenous access is not possible and/or not appropriate for all clinical settings. We currently use prosthetic accesses for the patients without autogenous options on the preoperative imaging, those with limited life expectancy, and those with marginal autogenous access options with previous autogenous access attempts that have not matured sufficiently for cannulation.
Determination of Autogenous Access Configuration
General Principles
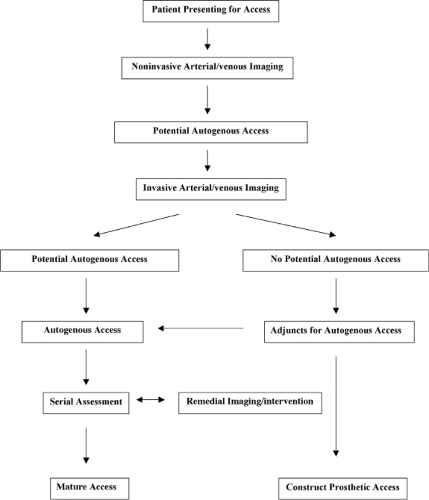
The overwhelming majority of patients presenting for permanent access are candidates for an autogenous access. Our approach, designed to optimize the use of autogenous upper extremity accesses, is predicated upon the standard principles of vascular surgery including adequate arterial inflow, adequate venous outflow, and a suitable conduit. Furthermore, it is based upon the use of dialysis catheters as a “bridge” or temporary access until the permanent access is suitable for cannulation and an aggressive approach to “failing” or “non-matured” accesses. Despite our use of the catheters as a “bridge,” we have made every attempt to limit the use these catheters, in accordance with the national guidelines, because of their acute infections complications and longer-term complications in terms of central vein stenoses and occlusions. See Figure 3.
History/Physical Examination
The initial evaluation of patents presenting for permanent hemodialysis access includes a focused history and physical examination. Special attention should be directed at documenting the access history including procedures, revisions, and associated complications. This should include any history of central vein cannulation, arm edema, and hand ischemia. Physical examination should include a detailed pulse examination with an Allen’s test to determine the forearm vessel responsible for the dominant arterial supply to the hand and examination of the neck and chest to look for venous collaterals.
Non-Invasive Imaging
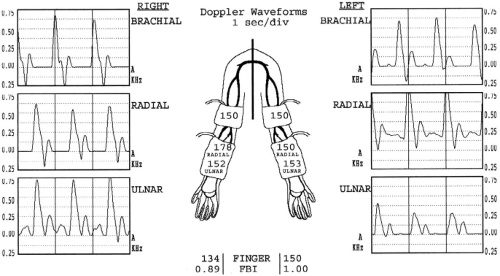
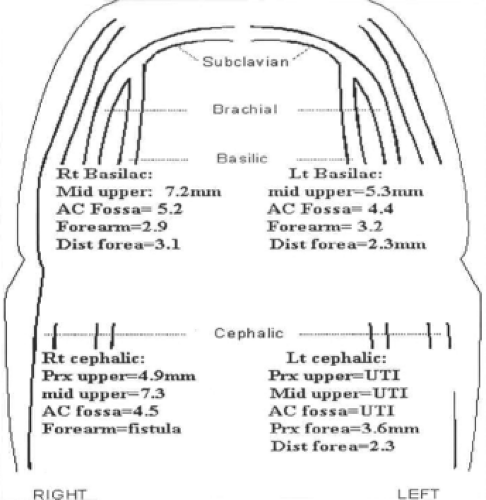
Non-invasive testing in the diagnostic vascular laboratory is the cornerstone of our algorithm. The examinations involve interrogation of both the arterial and venous circulation. The arterial studies include blood pressure measurements of the brachial, radial, ulnar, and digital arteries along with the corresponding Doppler waveforms of all but the digital vessels (Fig. 4). Additionally, the Allen’s test is repeated and the diameters of both the radial (at the wrist) and brachial (at the antecubital fossa) arteries are measured. Venous imaging includes the interrogation of the cephalic and basilic veins from the wrist to the axilla complete with diameter measurements similar to the preoperative vein survey obtained prior to infrainguinal arterial revascularization (Fig. 5). Additionally, the upper extremity and central veins are examined for the presence of deep venous thrombosis, although the interrogation of the veins within the thoracic cavity is limited.
Potential Autogenous Access Configuration
A preliminary operative plan is then generated based upon the results of the history/physical and non-invasive imaging. Our objective has been to select the combination of artery and vein that would most likely result in a successful autogenous access. We have not felt constrained by the usual conventions of using the non-dominant more than dominant extremity and the forearm more than arm, although we have followed these standard approaches when the choices are equivocal. The criteria for an adequate artery and vein include no hemodynamically significant arterial inflow stenoses, no venous outflow stenoses, and a peripheral vein segment of suitable length and diameter (Table 2). Our preferences in descending order include the radiocephalic, radiobasilic, brachiocephalic, and brachiobasilic autogenous accesses prior to the use of prosthetic material (Table 3). Notably, these preferences are consistent with the current KDOQI guidelines. We have had particularly good results with the autogenous brachiobasilic configuration. Indeed, the basilic vein is an excellent conduit for an autogenous access since it is usually relatively thick walled, large in diameter, and well preserved in terms of cannulation for venipunctures and intravenous catheters due to its relatively deep course.
Additional Imaging
Contrast arteriography and venography can be used to confirm the preliminary access choice. Although we have previously used these modalities more routinely, we now reserve them for select indications. The potential indications for arteriography (i.e., catheter-based or CT arteriography) include the presence of peripheral arterial occlusive disease, diabetes mellitus, complex access
procedures, multiple failed access procedures, abnormal noninvasive studies, and prior episodes of hand ischemia. The potential indications for venography (i.e., catheter-based or CT venography) include arm edema, multiple venous collaterals, pacemakers, multiple central venous catheters, multiple prior access procedures, and complex reconstructions. A catheter-based venogram can be performed simply by cannulating a superficial vein in the hand with an 18- or 20-gauge intravenous catheter and injecting a bolus of 20 mL of contrast followed by an equal volume of saline. Hemodynamically significant stenoses are suggested by the presence of collaterals, but can be confirmed by measuring intraluminal pressures across the lesion. Unfortunately, the catheter-based venogram has not been particularly helpful as a means to interrogate the more superficial basilic and cephalic veins. An ipsilateral catheter-based arteriogram can be performed if no central vein problems are identified. The arteriogram is performed using a retrograde femoral approach with complete visualization of the arterial tree from the aortic arch to the digits. This requires a flush catheter arteriogram of the thoracic aortic arch followed by selective cannulation of the extremity and contrast angiography with the arm extended in anatomic position. If a significant central vein problem is identified, a venogram on the contralateral extremity is performed prior to proceeding with the arteriogram. Endovascular treatment, either angioplasty alone or in combination with an intraluminal stent, may be performed at the same time of the invasive studies or at the time of the access procedure itself. However, the decision to proceed with intervention is contingent upon the clinical scenario and the other potential access options are identified by the non-invasive testing. Iodinated contrast is nephrotoxic and relatively contraindicated in the patients with chronic kidney disease (i.e., those patients not on dialysis). The contrast-associated nephrotoxicity can be reduced with a combination of hydration, acetylcysteine, and sodium bicarbonate. Unfortunately, the alternative contrast agent gadolinium is absolutely contraindicated for the patients with chronic kidney disease and ESRD because of the risk of nephrogenic systemic fibrosis. Similarly, the use of carbon dioxide in the upper extremity is also contraindicated because of the potential to enter the cerebral circulation. In the event that the invasive imaging does not support the initial access configuration, a second choice is made based upon the same principles (i.e., artery/vein requirements, imaging studies, hierarchy of procedures, conventions).
procedures, multiple failed access procedures, abnormal noninvasive studies, and prior episodes of hand ischemia. The potential indications for venography (i.e., catheter-based or CT venography) include arm edema, multiple venous collaterals, pacemakers, multiple central venous catheters, multiple prior access procedures, and complex reconstructions. A catheter-based venogram can be performed simply by cannulating a superficial vein in the hand with an 18- or 20-gauge intravenous catheter and injecting a bolus of 20 mL of contrast followed by an equal volume of saline. Hemodynamically significant stenoses are suggested by the presence of collaterals, but can be confirmed by measuring intraluminal pressures across the lesion. Unfortunately, the catheter-based venogram has not been particularly helpful as a means to interrogate the more superficial basilic and cephalic veins. An ipsilateral catheter-based arteriogram can be performed if no central vein problems are identified. The arteriogram is performed using a retrograde femoral approach with complete visualization of the arterial tree from the aortic arch to the digits. This requires a flush catheter arteriogram of the thoracic aortic arch followed by selective cannulation of the extremity and contrast angiography with the arm extended in anatomic position. If a significant central vein problem is identified, a venogram on the contralateral extremity is performed prior to proceeding with the arteriogram. Endovascular treatment, either angioplasty alone or in combination with an intraluminal stent, may be performed at the same time of the invasive studies or at the time of the access procedure itself. However, the decision to proceed with intervention is contingent upon the clinical scenario and the other potential access options are identified by the non-invasive testing. Iodinated contrast is nephrotoxic and relatively contraindicated in the patients with chronic kidney disease (i.e., those patients not on dialysis). The contrast-associated nephrotoxicity can be reduced with a combination of hydration, acetylcysteine, and sodium bicarbonate. Unfortunately, the alternative contrast agent gadolinium is absolutely contraindicated for the patients with chronic kidney disease and ESRD because of the risk of nephrogenic systemic fibrosis. Similarly, the use of carbon dioxide in the upper extremity is also contraindicated because of the potential to enter the cerebral circulation. In the event that the invasive imaging does not support the initial access configuration, a second choice is made based upon the same principles (i.e., artery/vein requirements, imaging studies, hierarchy of procedures, conventions).
Table 2 Criteria to Determine Suitability of Artery and Vein for Autogenous Access | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Table 3 Hierarchy for Permanent Hemodialysis Accesses Configurations | |||||||
|---|---|---|---|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree