FIGURE 41-1 Electrocardiogram during atrial tachycardia (AT) that occurred following catheter ablation of atrial fibrillation. The lack of a “saw-tooth” morphology of the P waves in the inferior leads and the presence of isoelectric P waves in the lateral precordial leads point to a left atrial (LA) origin. Also, note the notching (*) after the initial negative deflection (arrow).
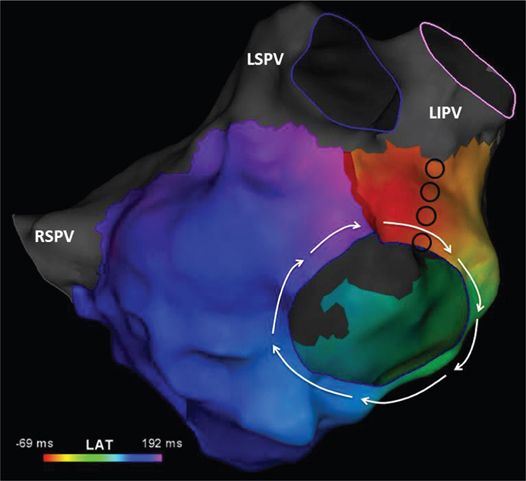
FIGURE 41-2 Activation map of the LA constructed using more than 500 points suggests that the mechanism of the AT (cycle length = 265 ms) is macroreentry around the mitral valve in a clockwise direction. The color spectrum (261 ms) shows that mapping accounted for almost the entire cycle length of the tachycardia, confirming a macroreentrant mechanism. The appendage (LAA) has been removed for clarity. Abbreviations: LI, left inferior; LS, left superior; PV, pulmonary vein; RS, right superior.
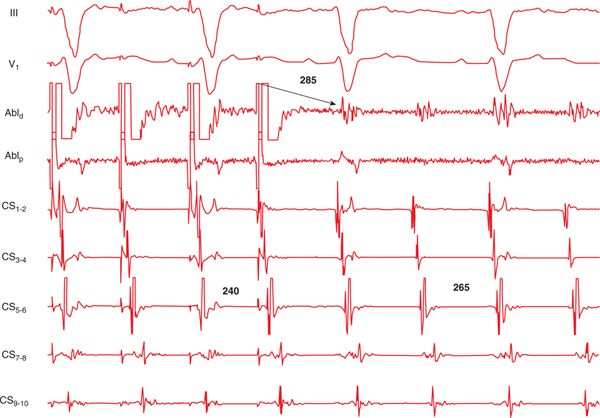
FIGURE 41-3 Entrainment mapping from the mitral isthmus (region between the lateral mitral annulus and the left inferior PV). The tachycardia is accelerated to the pacing rate, and the postpacing interval upon cessation of pacing approximates the cycle length of the tachycardia, confirming the findings on activation mapping shown in Figure 41-2. Abbreviations: Abl, ablation; CS, coronary sinus.
EPIDEMIOLOGY
Patients undergoing catheter ablation of atrial fibrillation (AF) may develop atrial tachycardia (AT) during follow-up.1,2 The incidence of postablation AT depends on a number of factors, including the underlying atrial substrate, the ablation approach, and possibly treatment with antiarrhythmic medications. In patients undergoing only pulmonary vein isolation, the incidence of postablation AT is low.3 Patients who undergo empiric linear ablation, the incidence is about 33%,2 with about one-fourth of the patients requiring a repeat ablation procedure for AT. AT is quite common when a step-wise approach is utilized in an attempt to acutely terminate AF during radiofrequency ablation. In fact, about 40% to 50% of such patients require a repeat ablation for AT.4
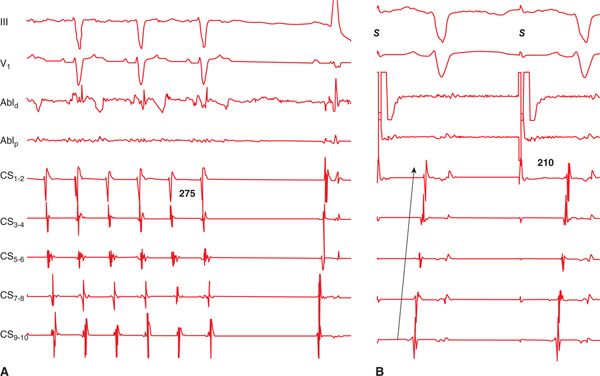
FIGURE 41-4 (A) Termination of mitral isthmus-dependent flutter during radiofrequency energy delivery along the line (black circles in Figure 41-2). (B) Pacing anterior to the line (from the region of the LAA) results in a proximal-to-distal activation of the coronary sinus, confirming the presence of linear block at the mitral isthmus. Abbreviation: S, stimulus.
ETIOLOGY AND PATHOPHYSIOLOGY
Broadly speaking, there are two main hypotheses that help explain the emergence of AT after catheter ablation of AF.5 It is possible that postablation AT represents an underlying “driver” that is only unmasked after eliminating AF or more precisely, fibrillatory conduction. A prior study using spectral analysis showed that the frequency (ie, activation rate) of the resultant AT after elimination of AF matched the frequency of a spectral component that was present during the initial periodogram during AF.6 This finding suggests that the substrate for AT—macroreentrant in the majority—is already present during AF and that the AT is destined to be encountered at some point. Although this hypothesis is certainly plausible, it may not be applicable to all patients. The other hypothesis posits that postablation AT is a result of the ablation approach, that is, a proarrhythmic complication of the initial AF procedure. There is a wealth of data that supports this contention. First, it is well known that both antral PVI and circumferential PV ablation (ie, circular lesions around the PVs, along with linear lesions at the mitral isthmus and the posterior wall/roof) are very effective in eliminating AF in patients with paroxysmal AF.7 However, the former does so without intervening macroreentrant AT, which is quite common with the latter approach. So if patients were destined to develop macroreentrant AT, why should they only do so with one technique and not both? In fact, one could argue that they should be less likely to develop macroreentrant AT with an approach that involves linear lesions than with one that does not. Second, a recent study showed that the area of slowest conduction velocity, requisite for the development of macroreentry, during either roof or perimitral flutter was found at the precise location where ablation was performed during the initial AF procedure.8 In other words, the area of slowest velocity during a roof- or mitral-isthmus-dependent AT was identified at the most cranial aspect of the roof, and the lateral mitral annulus, respectively. If macroreentrant AT were due to the underlying substrate, why would the area of slowest conduction be confined to the site that was ablated, and not other sites along the reentrant path? When sources of AF are mapped, as opposed to an empiric lesion set for all patients, ablation of AF often yields sinus rhythm without an intervening atrial tachycardia.9 Lastly, a prior study showed that >95% reentrant AT following circumferential PV ablation arose from the prior ablation targets/lines for AF.10 Therefore, AT in most patients is likely related to a proarrhythmic effect of ablation.
DIAGNOSIS
Establishing a diagnosis of postablation AT is straightforward. The diagnosis can be made on the ECG or ambulatory monitoring, or by analysis of stored electrograms in the patient with an implantable device. The ECG shows evidence of organized atrial activity, and that the P or flutter waves are identical with a consistent cycle length. This may require careful analysis of the ECG to distinguish between organized AF and AT. If the P waves are even slightly dissimilar in rate or appearance, the rhythm is likely AF and not AT. Not infrequently, the P waves may not be apparent due to low amplitude or are obscured by the QRS or T waves during rapid ventricular rates. An ECG during postablation AT is sometimes misdiagnosed as sinus tachycardia. A closer inspection of the ECG usually shows a P wave that is obscured by the QRS/T wave. Resting sinus tachycardia, in the absence of a coexisting illness in these patients—recall that patients with persistent AF have evidence of structural and sinus node remodeling—is uncommon.
The ECG may also be helpful in elucidating the mechanism of the atrial tachycardia. The P-wave duration during macroreentrant tachycardias (ie, “atrial flutter”) is longer owing to continuous atrial activation. A recent study suggested that a cut-off value >185 ms is consistent with macroreentry.8 During focal tachycardias or in situations in which reentry is confined to a small region of the atrium (ie, localized reentry or small reentrant circuits), the activation time is shorter, and the diastolic interval is longer as compared to large circuits. These features result in a shorter P-wave duration and a presence of an isoelectric interval between successive P waves. It should be stressed that in atria with multiple areas of conduction block or extensive scarring, these observations may not be applicable.
MANAGEMENT
Preprocedure Preparation
Not all patients with post-AF atrial tachycardia need to undergo a repeat ablation procedure. It is not unreasonable to perform transthoracic cardioversion (for persistent AT) or recommend antiarrhythmic medications (for paroxysmal AT) in the acute phase (within the first 3 months) following the AF procedure. In some of these patients, AT may not recur during long-term follow-up.2 For AT that occurs after 3 months of the AF procedure, our practice is to recommend catheter ablation. Note that most of these patients were referred to for the initial AF procedure after failure or intolerance of antiarrhythmic medications. Thus, to prescribe antiarrhythmic medications on an indefinite basis is not an attractive option.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree