Fig. 12.1 Some aspects of the early and late-phase responses in asthma.
Crosslinking of the over-expressed IgE on mast cells of atopic individuals, and non-immunogenic stimuli in more severe non-atopic asthma, can degranulate mast cells, resulting in secretion of mediators that contribute to the pathogenesis of asthma. These mediators directly produce bronchoconstriction, initiate the acute inflammatory response, and attract and activate cells responsible for further inflammatory mediator production and persistent chronic inflammation. LT, leukotriene; PG, prostaglandin.
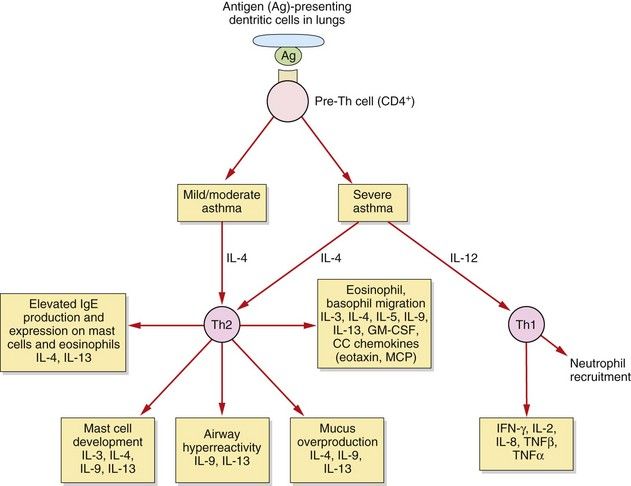
Fig. 12.2 T-cells and asthma.
In allergic asthma there are complex and still poorly understood imbalances in the immune system. These include alterations in the functioning of several T-cell subsets and additional dysregulation in epithelial cells, fibroblasts and airway dendritic cells. In mild to moderate allergic asthma, the T-helper cell type 2 (Th2) response is amplified, and Th2 cytokines contribute to many of the pathophysiological features of asthma. In severe asthma there is an additional pathological role for T-helper type 1 (Th1) cytokines and neutrophils. IL, interleukin; GM-CSF, granulocyte–macrophage colony-stimulating factor; IFN, interferon; MCP, monocyte chemoattractant proteins, TNF, tumour necrosis factor.
Figure 12.1 shows how exposure of atopic individuals to a relevant allergen (such as pollen or the faeces of house-dust mite) crosslinks IgE bound to mast cell membrane receptors and causes mast cell degranulation. Degranulation and the subsequent pathological processes can also occur in hypersensitive non-atopic asthmatics with normal levels of IgE, triggered by other factors such as upper respiratory tract infections, particularly with human rhinovirus. Degranulation of mast cells produces immediate bronchoconstriction (early phase) due to the release of a number of spasmogens, of which the most potent are cysteinyl-leukotrienes (Fig. 12.1). Chemotactic mediators are also released, promoting an influx of inflammatory cells which 4–6 h later results in a delayed bronchoconstrictor response (late-phase) and the commencement of a cascade of other pathological events in the airways. The persistent release of spasmogens and inflammatory mediators by these infiltrating cells can leave the bronchi hyperreactive to various irritants for several weeks. The inflammatory mediators produce mucosal oedema, which narrows the airways, and stimulate smooth muscle contraction leading to bronchoconstriction. Excessive production of mucus can cause further airways obstruction by plugging the bronchiolar lumen.
In mild to moderate asthma there is an increase in the number and activation of eosinophils (accompanied by some neutrophils and macrophages) in the airway and hyperresponsiveness of the airways to irritants and spasmogens. There is a persistent and excessive T-helper cell type 2 (Th2) immune response (Fig. 12.2). All airways are involved in the inflammatory process in mild to moderate asthma, but the degree of submucosal fibrosis and mucus secretion is modest, with no parenchymal destruction.
In severe asthma there is evidence of additional, greater infiltration of neutrophils, tissue destruction and airways remodelling, with progressive thickening and loss of elastic recoil, especially in the peripheral airways. In addition to the changes seen in mild to moderate asthma, in severe disease there is increased expression of T-helper cell type 1 (Th1)-derived cytokines.
Chronic obstructive pulmonary disease
About 95% of people with COPD are, or have been, cigarette smokers. There is wide variability in the rate of decline in pulmonary function in persistent smokers, with about 10–20% showing an accelerated decline that may reflect a genetic susceptibility. Less common causes of COPD are exposure to air pollution (including biomass fuels in the developing world) and inherited α1-antiprotease deficiency.
COPD is a symptom complex that is characterised by persistent airflow obstruction, with most people showing limited reversibility in response to a bronchodilator; however, about 10% of people with COPD do show considerable bronchodilator-induced reversibility of the airflow obstruction, and have a mixed inflammatory pattern in the airways, which probably represents an overlap between asthma and COPD (wheezy bronchitis). The airflow obstruction in COPD is usually slowly progressive and results from a combination of decreased bronchial luminal diameter (produced by wall thickening, intraluminal mucus and changes in the fluid lining the small airways) and dynamic airways collapse due to emphysema (see below). It is often accompanied by chronic bronchitis (production of mucoid sputum for all or part of the year).
The most frequent symptoms of COPD are gradually progressive breathlessness and cough. The cough is often productive and usually worse in the morning, but its severity is unrelated to the degree of airflow obstruction. Repeated respiratory infections are common, and are often associated with exacerbations of the airflow obstruction and symptomatic deterioration.
In COPD there is an inflammatory process that particularly affects the peripheral airways. The predominant infiltrating cells are neutrophils and macrophages, but ongoing damage to the lung even after the trigger is removed is probably due to T-lymphocyte-mediated inflammation (Fig. 12.3). There is increased oxidative stress due to reactive oxygen species derived from cigarette smoke and other pollutants and released from neutrophils and inflammatory macrophages. The inflammation produces a marked fibrotic reaction with parenchymal destruction and excessive bronchial mucus secretion. Corresponding histological changes include an increase in goblet cells in the bronchial mucosa and an increase in muscle mass in the bronchial wall, accompanied by interstitial fibrosis. Apoptosis of endothelial and alveolar cells reduces the ability of the lung to repair itself in response to sustained injury from the inhaled pollutants.
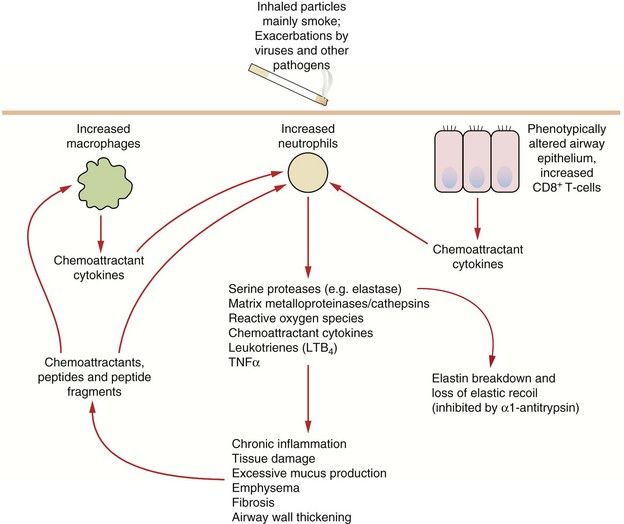
Fig. 12.3 Some pathophysiological factors in COPD.
A small percentage of people who smoke are particularly susceptible to the development of COPD; susceptibility may be determined by variability in inflammatory or protective genes. Chronic alterations in the recruitment, activation and control in function of neutrophils, macrophages and subsets of T-cells results in chronic parenchymal damage, loss of elastic recoil and episodes of infection. TNF, tumour necrosis factor.
Emphysema is a pathological description, and is defined as enlargement of airways distal to the terminal bronchioles owing to destructive changes that may involve the entire acinus (panacinar) or the central part of the acinus (centriacinar). Lung parenchymal destruction is largely mediated by tissue proteases and cathepsins that are released by neutrophils and macrophages. Generation of excessive amounts of reactive oxygen species inhibits the antiproteases that normally protect the lung against such attack. This explains the susceptibility of people with inherited α1-antiprotease deficiency to emphysema. Tissue destruction leads to a loss of lung recoil on expiration, which is a major factor in generating expiratory flow. Emphysema is probably the dominant factor in severe COPD.
Drugs for asthma and chronic obstructive pulmonary disease
For the treatment of airways disease, direct delivery of drug to the lung by inhalation allows the use of smaller doses and therefore reduces the incidence of unwanted systemic effects (see Table 12.1). It also allows rapid onset of action of ‘rescue’ medication. The drug is usually delivered to the airways in an aerosol. The size of the aerosol particle that is inhaled is an important factor that determines whether or not it will reach the airways and where in the airways it will be deposited. The optimal particle size for treatment is 2–5 µm. Particles larger than 10 µm impact on the upper airways and will be swallowed. Particles smaller than 1 µm will not be deposited in the lower respiratory tract, and will either reach the alveoli and are absorbed into the blood or are exhaled. Other factors that influence aerosol deposition include the pattern of inhalation, the properties of the carrier and the type and severity of the lung disease. There are several methods for delivery of inhaled drug.
Table 12.1
Comparison of aerosol and oral therapy for asthma
| Aerosol | Oral | |
| Ideal pharmacokinetics | Slow absorption from the lung surface and rapid systemic clearance | Good oral absorption and slow systemic clearance |
| Dose | Low dose delivered rapidly to target | High systemic dose necessary to achieve an appropriate concentration in the lung |
| Systemic drug concentration | Low | High |
| Incidence of unwanted effects | Low | High (but depends on drug) |
| Distribution in the lung | Reduced in severe disease | Unaffected by disease |
| Compliance | Good with bronchodilators, poor with anti-inflammatory drugs | Good |
| Ease of administration | Difficult for small children and infirm peoplea | Good |
| Effectiveness | Good in mild to moderate disease | Good even in severe disease |
aMay be improved by breath-actuated inhalers or spacing devices. Nebulisers can be used for severe exacerbations.
Pressurised metered-dose inhaler
This is the most common device for delivery of bronchodilator and anti-inflammatory drugs used in the treatment of asthma and COPD. The propellant in the device is a pressurised hydrofluoroalkane (HFA) (having replaced chloroflurocarbons, or CFCs) and activation delivers a measured dose of aerosol via an atomization nozzle. Manually activated inhalers are widely used since they are convenient and inexpensive, but they require coordination of device activation and inhalation. The delivery and uptake of the drug are suboptimal if inspiratory flow is low, if inspiration is not full and is not preceded by full expiration, or if inspiration is followed by a breath hold of less than 6 s. About one-third of users find coordination difficult and, even if it is optimal, up to 70–90% of the aerosol may be deposited in the oropharynx and swallowed. The inhaler should be shaken before use.
Pressurised metered-dose inhaler with a spacer
A spacer device (a plastic reservoir) can be attached to the pressurised metered-dose inhaler to act as a chamber from which the suspended aerosol particles can be inhaled. The use of a spacer removes the need to coordinate aerosol activation and inspiration. A spacer can be large volume which retains more of the aerosol, or small volume which is more convenient but in which the aerosol impacts to a greater extent on the wall of the spacer. The spacer can be designed as a holding chamber by incorporating a one-way valve that retains the aerosol in the chamber for longer. A spacer is essential for young children, and for very young children a holding chamber can be attached to a facemask. The inhaler is activated into the spacer, and the person breathes normally through the mouthpiece. Inhalation of the contents should be completed within 10 s. The spacer allows evaporation of propellant and may create more droplets of the correct size to deposit in the airways. It also reduces drug deposition in the oropharynx, due to reduced particle velocity. Electrostatic charge on the plastic wall can attract particles and reduce drug delivery, so non-electrostatic materials are preferred. The device should be washed in mild detergent and air-dried to minimise the electrostatic charge. Addition of a spacer makes a metered-dose inhaler system less portable and may reduce adherence with treatment.
Breath-actuated metered-dose inhaler
There are several types of breath-actuated metered-dose inhaler, delivering either an aerosol or dry powder. The aerosol type is a modified metered-dose inhaler that is activated by inspiration. Actuation requires air to be drawn through the mouthpiece at a flow rate of at least 30 L⋅min−1. People who have severe airflow obstruction cannot achieve this. Breath-actuated metered-dose inhaler devices cannot be used with a spacer.
Dry-powder inhaler
Dry-powder inhalers contain particles of drug of optimal size for deposition. Inspiration through the device generates turbulence, which disperses the particles in the inspired air. Some devices use a single dose capsule, while in others the source is a bulk powder with the device metering the dose. The delivered dose is dependent on the inspiratory effort, unless the device is power-assisted by a battery or vibrating piezoelectric crystals.
Multi-dose liquid inhaler
This novel delivery device uses a spring to force a metered dose of liquid through a narrow nozzle. It creates more fine particles and gives high drug delivery to the lungs.
Nebulisers
Nebulisers are devices that are used with a facemask or mouthpiece to deliver drug from a reservoir solution. There are two types.
 Jet nebulisers use compressed air or oxygen passing through a narrow orifice at 6–8 L⋅min−1 to suck drug solution from a reservoir into a feed tube. There are fine ligaments in this tube, and the impact of the solution on these ligaments generates droplets (Venturi principle). Baffles trap the larger droplets.
Jet nebulisers use compressed air or oxygen passing through a narrow orifice at 6–8 L⋅min−1 to suck drug solution from a reservoir into a feed tube. There are fine ligaments in this tube, and the impact of the solution on these ligaments generates droplets (Venturi principle). Baffles trap the larger droplets. Ultrasonic nebulisers use a piezoelectric crystal vibrating at high frequency to create the aerosol, and do not require gas flow. The vibrations are transmitted through a buffer to the drug solution and form a fountain of liquid in the nebulisation chamber. Ultrasonic nebulisers produce a more uniform particle size than jet nebulisers, but are less widely used due to cost.
Ultrasonic nebulisers use a piezoelectric crystal vibrating at high frequency to create the aerosol, and do not require gas flow. The vibrations are transmitted through a buffer to the drug solution and form a fountain of liquid in the nebulisation chamber. Ultrasonic nebulisers produce a more uniform particle size than jet nebulisers, but are less widely used due to cost.Up to 10 times the amount of drug is required in a nebuliser to produce the same degree of bronchodilation achieved by a metered-dose inhaler. Drug delivery is more efficient via a mouthpiece than via a mask from which drug can be deposited in the nasal passages.
Symptom-relieving drugs for airflow obstruction (bronchodilators; ‘relievers’)
Mechanism of action and effects: Beta2-adrenoceptors are widely distributed in the lung, and the receptor density is higher in bronchial smooth muscle than in other cell types such as epithelial and endothelial cells and mast cells. Stimulation of these receptors by an agonist stabilises the receptor in its active rather than inactive configuration. This results in increased generation of cAMP by adenylyl cyclase, and activation of protein kinase A (PKA), which phosphorylates proteins that are central to the regulation of smooth muscle tone. Major beneficial actions of a β2-adrenoceptor agonist are:
 bronchodilation due to reduced Ca2+ release from intracellular stores and reduced Ca2+ entry into smooth muscle cells,
bronchodilation due to reduced Ca2+ release from intracellular stores and reduced Ca2+ entry into smooth muscle cells,However, in addition to their beneficial effects on the airway, use of a β2-adrenoceptor agonist in asthma can enhance Th2 inflammatory pathways and also downregulate β2-adrenoceptors. Therefore, regular use of a β2-adrenoceptor agonist without an inhaled corticosteroid is not advised. There is evidence of synergy between inhaled corticosteroids and inhaled β2-adrenoceptor agonists, with the latter enhancing the gene-transcription effects of corticosteroids and corticosteroids enhancing β2-adrenoceptor gene transcription.
Some β2-adrenoceptor agonists, such as salbutamol, terbutaline and salmeterol, have about 60% partial agonist activity at the receptor (low-efficacy agonists) compared with formoterol and indacaterol which have full agonist activity (high-efficacy agonists). The relevance of these differences to treatment outcomes and unwanted effects is unclear.
Pharmacokinetics: The selectivity of β2-adrenoceptor agonists for the β2-adrenoceptor subtype is dose-dependent. Inhalation of drug aids selectivity since it delivers small but effective doses to the airways and minimises systemic exposure and stimulation of β-adrenoceptors outside the lungs (Table 12.1). The dose–response relationship for bronchodilation is log-linear and a 10-fold increase in dose is required to double the effect.
Short-acting β2-adrenoceptor agonists, such as salbutamol, have a rapid onset of action, often within 5 min, and produce bronchodilation for up to about 6 h. Their duration of action is far longer than the natural adrenoceptor agonists such as adrenaline, because they are not substrates for the uptake transporter on the presynaptic neuron or for catechol-O-methyltransferase, the enzyme that metabolises catecholamines outside adrenergic neurons (Ch. 4).
Salmeterol and formoterol have a longer duration of action (up to 12 h) because they are more lipophilic than short-acting agents and bind to the lipid of the cell membrane. Salmeterol has a slower onset of action than short-acting agents, but the onset with formoterol is rapid. Indacaterol is an ultra-long acting (up to 24 h) lipophilic, rapid-onset β2-adrenoceptor agonist.
Salbutamol and terbutaline can also be given orally (as conventional or modified-release formulations), by subcutaneous or intramuscular injection or by intravenous infusion. Much larger doses are required to deliver an adequate amount of drug to the lungs by any of these routes compared to inhaled doses. This reduces the selectivity for β2-adrenoceptors, and systemic unwanted effects can be troublesome.
 Tachycardia and arrhythmias result from both β1– and β2-adrenoceptor stimulation in the heart when high doses of inhaled drug are used, or after oral or parenteral administration.
Tachycardia and arrhythmias result from both β1– and β2-adrenoceptor stimulation in the heart when high doses of inhaled drug are used, or after oral or parenteral administration. Hypokalaemia with high doses, due to promotion of cellular uptake of K+ by a cAMP-dependent action of β2-adrenoceptor agonists on the Na+/K+ pump. Nebilised salbutamol is sometimes used as a treatment for hyperkalaemia. Hypomagnesaemia and hyperglycaemia can also occur. These effects do not persist during long-term use.
Hypokalaemia with high doses, due to promotion of cellular uptake of K+ by a cAMP-dependent action of β2-adrenoceptor agonists on the Na+/K+ pump. Nebilised salbutamol is sometimes used as a treatment for hyperkalaemia. Hypomagnesaemia and hyperglycaemia can also occur. These effects do not persist during long-term use. Paradoxical bronchospasm has been reported with inhalation, usually when given for the first time or with a new canister.
Paradoxical bronchospasm has been reported with inhalation, usually when given for the first time or with a new canister. Tolerance to the bronchodilator effects with prolonged use of β2-adrenoceptor agonists is modest, but desensitisation and downregulation of the β2-adrenoceptor does occur. The process of receptor desensitisation appears to be more rapid for mast cells than for bronchial smooth muscle, and the prevention of exercise-induced bronchoconstriction is more affected than the symptom relief that these drugs produce. Corticosteroids reduce desensitisation by increasing β2-adrenoceptor gene transcription and enhancing coupling of the receptor to adenylyl cyclase.
Tolerance to the bronchodilator effects with prolonged use of β2-adrenoceptor agonists is modest, but desensitisation and downregulation of the β2-adrenoceptor does occur. The process of receptor desensitisation appears to be more rapid for mast cells than for bronchial smooth muscle, and the prevention of exercise-induced bronchoconstriction is more affected than the symptom relief that these drugs produce. Corticosteroids reduce desensitisation by increasing β2-adrenoceptor gene transcription and enhancing coupling of the receptor to adenylyl cyclase.Regular use of high doses of short-acting or inhaled long-acting β2-adrenoceptor agonists has been linked with asthma deaths. One possibility is that they precipitate serious cardiac arrhythmias during severe asthma exacerbations. It is also possible that their use might allow people to tolerate initial exposure to larger doses of allergens or irritants, which then produce an enhanced late asthmatic response. The excess mortality, although of concern, is extremely low. Recent investigation has raised the possibility that β2-adrenoceptor polymorphism may modify the response to β2-adrenoceptor agonists in some individuals, but it is not known whether this explains the risk of adverse events.
Antimuscarinic agents
Mechanism of action and effects: Many cell types in the respiratory system, including both neuronal and non-neuronal cells, have nicotinic and muscarinic surface receptors. These mediate a multitude of actions in response to parasympathetic nervous system stimulation. There are two main types of muscarinic receptors in the airways, as follows.
 M3 receptors mediate direct bronchoconstriction and glandular mucus secretion and also enhance mucociliary clearance from the bronchi. M3 receptor stimulation activates phospholipase C with subsequent formation of inositol triphosphate (IP3) and diacylglycerol (DAG), which are key events in the signalling pathway that increases intracellular Ca2+ (Ch. 1, Fig. 1.5).
M3 receptors mediate direct bronchoconstriction and glandular mucus secretion and also enhance mucociliary clearance from the bronchi. M3 receptor stimulation activates phospholipase C with subsequent formation of inositol triphosphate (IP3) and diacylglycerol (DAG), which are key events in the signalling pathway that increases intracellular Ca2+ (Ch. 1, Fig. 1.5).Therefore, blocking both M2 and M3 receptors could be beneficial in bronchoconstriction. However, M2 autoreceptors are also present on presynaptic parasympathetic nerves supplying the lungs. Stimulation of these autoreceptors inhibits acetylcholine release and attenuates vagally mediated bronchoconstriction. Blocking these M2 autoreceptors may blunt the beneficial effect of non-selective muscarinic antagonists.
Ipratropium is a non-selective muscarinic receptor antagonist, and binds to all muscarinic receptors in the lung including the presynaptic M2 autoreceptor. Ipratropium therefore has the potential to augment vagally mediated bronchoconstriction. The recommended dose is determined by unwanted effects and is well below the dose that produces maximal bronchodilation. By contrast, tiotropium is functionally selective for the M3 receptor. Although it has a high affinity for all muscarinic receptors, it dissociates rapidly from M2 receptors.
The main benefit of muscarinic antagonists is in COPD; they are of less value for bronchodilation in acute mild to moderate asthma, but ipratropium has a place when added to a β2-adrenoceptor agonist in severe exacerbations of asthma.
Pharmacokinetics: The antimuscarinic drugs used for bronchodilation are N-quaternary congeners of the tertiary-structured atropine, and are poorly absorbed orally and do not cross the blood–brain barrier. They are given exclusively by inhalation as a powder or aerosol or via a nebuliser. They have a slower onset of action (30–60 min) than salbutamol (5–10 min), probably due to slow absorption from the surface of the airways. The duration of action is related to the rate of removal locally from the airways, and not the half-life of elimination from the circulation.
Methylxanthines
Mechanism of action and effects: Methylxanthines are a group of naturally occurring substances found in coffee, tea, chocolate and related foodstuffs. Naturally occurring theophylline (1,3-dimethylxanthine), and its ester derivative aminophylline, are the only compounds in clinical use. They are chemically similar to caffeine. Methylxanthines have vasodilatory, anti-inflammatory and immunomodulatory actions. The mechanisms of action of methylxanthines are multiple, controversial and of uncertain importance.
 Inhibition of the enzyme phosphodiesterase (PDE), which degrades cyclic nucleotide second messengers, may partly explain the actions of methylxanthines. Theophylline preferentially inhibits the isoenzymes PDE3 (which degrades cAMP and cGMP) and PDE4 (which degrades cAMP). PDE3 is found in bronchial smooth muscle and PDE4 in several inflammatory cell types, including mast cells. The rise in intracellular cAMP in bronchial smooth muscle stimulates large-conductance voltage-gated Ca2+-activated K+channels (BKCa) in the cell membrane, leading to cell hyperpolarisation and muscle relaxation. However, theophylline only produces bronchodilation at relatively high plasma concentrations (10–20 mg⋅L−1) and drugs that are more effective PDE inhibitors (such as dipyridamole) do not bronchodilate. Prolonging the duration of action of cyclic nucleotides may potentiate the action of β2-adrenoceptor agonists and produce a synergistic dilator effect on bronchial smooth muscle. PDE inhibition also stimulates ciliary beat frequency in the airways and enhances water transport across the airway epithelium, which increase mucociliary clearance. In contrast, theophylline increases the force and rate of contraction of cardiac muscle through its effect on cAMP (Ch. 7), but also causes arterial vasodilation by inhibiting the breakdown of cGMP.
Inhibition of the enzyme phosphodiesterase (PDE), which degrades cyclic nucleotide second messengers, may partly explain the actions of methylxanthines. Theophylline preferentially inhibits the isoenzymes PDE3 (which degrades cAMP and cGMP) and PDE4 (which degrades cAMP). PDE3 is found in bronchial smooth muscle and PDE4 in several inflammatory cell types, including mast cells. The rise in intracellular cAMP in bronchial smooth muscle stimulates large-conductance voltage-gated Ca2+-activated K+channels (BKCa) in the cell membrane, leading to cell hyperpolarisation and muscle relaxation. However, theophylline only produces bronchodilation at relatively high plasma concentrations (10–20 mg⋅L−1) and drugs that are more effective PDE inhibitors (such as dipyridamole) do not bronchodilate. Prolonging the duration of action of cyclic nucleotides may potentiate the action of β2-adrenoceptor agonists and produce a synergistic dilator effect on bronchial smooth muscle. PDE inhibition also stimulates ciliary beat frequency in the airways and enhances water transport across the airway epithelium, which increase mucociliary clearance. In contrast, theophylline increases the force and rate of contraction of cardiac muscle through its effect on cAMP (Ch. 7), but also causes arterial vasodilation by inhibiting the breakdown of cGMP. Increased diaphragmatic contractility and reduced fatigue have been reported at lower plasma theophylline concentrations than those required for bronchodilation. This may improve lung ventilation.
Increased diaphragmatic contractility and reduced fatigue have been reported at lower plasma theophylline concentrations than those required for bronchodilation. This may improve lung ventilation. Adenosine receptor antagonism may be relevant to some of the clinical effects of methylxanthines (see also adenosine; Ch. 8). Adenosine releases histamine and leukotrienes from mast cells, which results in the constriction of hyperresponsive airways in individuals with asthma. Theophylline is a potent antagonist at adenosine A1 and A2 receptors (Ch. 1) and may reduce bronchoconstriction by this mechanism. Adenosine receptor antagonism is responsible for central nervous system (CNS) stimulation, which improves mental performance and alertness, and in the kidney reduces tubular Na+ reabsorption and leads to natriuresis and diuresis.
Adenosine receptor antagonism may be relevant to some of the clinical effects of methylxanthines (see also adenosine; Ch. 8). Adenosine releases histamine and leukotrienes from mast cells, which results in the constriction of hyperresponsive airways in individuals with asthma. Theophylline is a potent antagonist at adenosine A1 and A2 receptors (Ch. 1) and may reduce bronchoconstriction by this mechanism. Adenosine receptor antagonism is responsible for central nervous system (CNS) stimulation, which improves mental performance and alertness, and in the kidney reduces tubular Na+ reabsorption and leads to natriuresis and diuresis. Activation of histone deacetylases (HDACs; see corticosteroids, below): acetylation of core histones, which form part of the structure of chromatin, activates gene transcription, while their deacetylation suppresses gene transcription, including transcription of pro-inflammatory genes. Theophylline at low concentrations activates HDAC in nuclear extracts, indicating an action independent of adenosine and other surface receptors, and also increases HDAC activity in bronchial biopsies of asthmatic patients. Anti-inflammatory effects of theophylline occur at drug plasma concentrations of 5–10 mg⋅L−1, similar to those that produce clinical benefit. The action of theophylline on HDAC may potentiate the anti-inflammatory effects of corticosteroids (see Ch. 44).
Activation of histone deacetylases (HDACs; see corticosteroids, below): acetylation of core histones, which form part of the structure of chromatin, activates gene transcription, while their deacetylation suppresses gene transcription, including transcription of pro-inflammatory genes. Theophylline at low concentrations activates HDAC in nuclear extracts, indicating an action independent of adenosine and other surface receptors, and also increases HDAC activity in bronchial biopsies of asthmatic patients. Anti-inflammatory effects of theophylline occur at drug plasma concentrations of 5–10 mg⋅L−1, similar to those that produce clinical benefit. The action of theophylline on HDAC may potentiate the anti-inflammatory effects of corticosteroids (see Ch. 44).Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree












