and Steven Novick2
(1)
GSK, Research Triangle Park, NC, USA
(2)
MedImmune, Gaithersburg, MD, USA
Abstract
Content Uniformity tests are used to establish that the dosage units of a drug product consistently contain the specified amount of drug (active pharmaceutical ingredient). The term uniformity may refer to uniformity within a batch, or within-product uniformity when evaluating multi-dose units such as inhaled and topical products. Various guidance documents regarding the establishment of content uniformity of drug product exist and are discussed in this chapter. In particular, we consider USP standards <905>, <3>, as well as the parametric tolerance interval test (PTIT). While the current version of USP <601> does not offer criteria to establish content uniformity, we discuss the method suggested in the 2011 proposed revision to the USP to illustrate the &zero tolerance’ approach to establishing content uniformity. While the current version of USP <601> does not offer criteria to establish content uniformity, we discuss the method suggested in the 2011 proposed revision to the USP to illustrate the ‘zero tolerance’ approach to establishing content uniformity. It is worth noting that there is much discussion within the industry and amongst regulatory agencies regarding the appropriateness of using the current USP standards to evaluate content uniformity. Consideration is being given to revision of these standards, and implementing alternatives such as the parametric tolerance interval test (PTIT) approach.
Keywords
USPParametric tolerance interval testCuDAL method24.1 U.S. Pharmacopeial Convention (USP) Standards for Content Uniformity
USP standards are often referenced for establishing content uniformity of a product. According to their website, the U.S. Pharmacopeial Convention (USP) “…is a scientific nonprofit organization that sets standards for the identity, strength, quality, and purity of medicines, food ingredients, and dietary supplements manufactured, distributed and consumed worldwide. USP’s drug standards are enforceable in the United States by the Food and Drug Administration, and these standards are used in more than 140 countries.”
This chapter will focus specifically on three USP standards: <905> Uniformity of Dosage Units, <3> Topical and Transdermal Drug Products—Product Quality Tests, and the zero tolerance approach in the 2011 suggested revisions to USP <601> Aerosols, Nasal Sprays, Metered-Dose Inhalers, and Dry Powder Inhalers. In general, standards <905>, the 2011 suggested revisions to <601> and <3> are two tiered procedures for testing content uniformity of a batch of drug product. They are implemented by selecting a set n = n 1 + n 2 dosage units for evaluation. For tier 1, the acceptance criteria are applied to n 1 of the dosage units. If the acceptance criteria are met, content uniformity is established under that USP standard. If not, the second set of n 2 dosage units may be evaluated. The second tier testing combines the results of the n 2 dosage units with those from tier 1 and then evaluates the n 1 + n 2 total dose units against a second set of acceptance criteria. The following three sections of this chapter will discuss the details and statistical properties of these USP standards.
It is worth noting that these tests are revised and updated over time; the latest USP chapter should be referenced to ensure the most up to date standard is utilized. The USP methods may not be harmonized with the European Pharmacopoeia and the Japanese Pharmacopoeia, and so those resources should be consulted when applicable. USP and other regulatory guidance documents may specify the method used to obtain content uniformity results (e.g. content uniformity, weight variation), and is beyond the scope of this chapter.
24.2 Process for Establishing Content Uniformity Using USP <905>
USP <905>, Uniformity of Dosage Units, applies to single dosage units (e.g., tablets), and is not intended to apply to suspensions, emulsions, or gels in unit-dose containers intended for external, cutaneous administration. The USP <905> content uniformity testing calls for a two-tiered testing approach where thirty dosage units are selected. Ten of these dosage units are assayed individually for tier 1 using the appropriate analytical method. If the ten tablets meet the acceptance criteria, then content uniformity has been established. If the tablets in this sample do not meet the acceptance criteria, the remaining 20 dosage units are assayed and evaluated in combination with the individual assay results from tier 1, using the criteria for tier 2. Note that the sample sizes in tier 1 and tier 2 are inflexibly fixed. The specific criteria are as follows:
1.
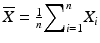 , and
, and ![$$ s={\left[\frac{{\displaystyle {\sum}_{i=1}^n}{\left({X}_i-\overline{X}\right)}^2}{n-1}\right]}^{\frac{1}{2}}. $$](/wp-content/uploads/2016/07/A330233_1_En_24_Chapter_IEq3.gif)
Calculate the mean (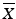 ) and standard deviation (s) of the assay results where n = 10 for tier 1, and n = 30 for tier 2,
) and standard deviation (s) of the assay results where n = 10 for tier 1, and n = 30 for tier 2,
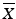 ) and standard deviation (s) of the assay results where n = 10 for tier 1, and n = 30 for tier 2,
) and standard deviation (s) of the assay results where n = 10 for tier 1, and n = 30 for tier 2,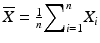 , and
, and ![$$ s={\left[\frac{{\displaystyle {\sum}_{i=1}^n}{\left({X}_i-\overline{X}\right)}^2}{n-1}\right]}^{\frac{1}{2}}. $$](/wp-content/uploads/2016/07/A330233_1_En_24_Chapter_IEq3.gif)
2.
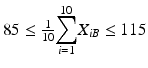 and
and 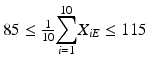 .
.
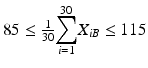 and
and 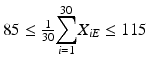 .
.
Get Clinical Tree app for offline access

Establish the Reference Value (M). This value depends on the target content (T) per dosage unit at the time of manufacture and the observed mean of the sample (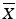 ). The value T is expressed as a percent of the label claim, and is typically 100 %.
). The value T is expressed as a percent of the label claim, and is typically 100 %.
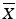 ). The value T is expressed as a percent of the label claim, and is typically 100 %.
). The value T is expressed as a percent of the label claim, and is typically 100 %.a.
Case 1: T ≤ 101.5
i.
If 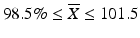 then M=
then M= 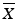
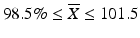 then M=
then M= 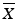
ii.
If 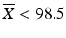 , then M = 98.5
, then M = 98.5
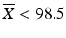 , then M = 98.5
, then M = 98.5iii.
If 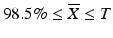 then M=
then M= 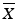
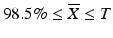 then M=
then M= 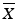
ii.
If 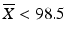 , then M = 98.5
, then M = 98.5
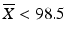 , then M = 98.5
, then M = 98.5iii.
If 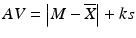
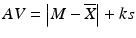
4.
The requirements for dosage uniformity are met if:
a.
Tier 1: The AV of the first ten dosage units is less than L 1 = 15.0 %. tier 2: If the AV for the first ten dosage units is greater than L 1 = 15.0 %, the criteria are met if (tier 2) the final acceptance value of the 30 dosage units is less than L 1 = 15.0 % and if all individual dosage units (X i, i = 1,…,30) meet the requirement ![$$ \left[1-(0.01)*{L}_2\right]*M\le {X}_i\le \left[1+(0.01)*{L}_2\right]*M $$](/wp-content/uploads/2016/07/A330233_1_En_24_Chapter_IEq13.gif) , where L 2 = 25.0 %.
, where L 2 = 25.0 %.
![$$ \left[1-(0.01)*{L}_2\right]*M\le {X}_i\le \left[1+(0.01)*{L}_2\right]*M $$](/wp-content/uploads/2016/07/A330233_1_En_24_Chapter_IEq13.gif) , where L 2 = 25.0 %.
, where L 2 = 25.0 %.Example
Ten capsules are assayed from a batch, and the following ten results are observed: {96.4, 104.9, 104, 103.5, 97.5, 92.4, 96.2, 107.8, 91.2, 100.2}. The target value (T) for this product is 100. The mean and standard deviation of these results are 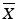 = 99.4 and s = 5.6, respectively. Since
= 99.4 and s = 5.6, respectively. Since 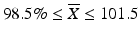 ,
, 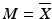 , and the AV is calculated as k*s = 2.4*5.6 = 13.44. Since this value is less than 15.0 (L 1), the criteria for establishing content uniformity using USP <905> are met.
, and the AV is calculated as k*s = 2.4*5.6 = 13.44. Since this value is less than 15.0 (L 1), the criteria for establishing content uniformity using USP <905> are met.
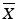 = 99.4 and s = 5.6, respectively. Since
= 99.4 and s = 5.6, respectively. Since 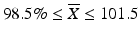 ,
, 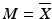 , and the AV is calculated as k*s = 2.4*5.6 = 13.44. Since this value is less than 15.0 (L 1), the criteria for establishing content uniformity using USP <905> are met.
, and the AV is calculated as k*s = 2.4*5.6 = 13.44. Since this value is less than 15.0 (L 1), the criteria for establishing content uniformity using USP <905> are met.24.2.1 Discussion USP <905>
Operating characteristic curves were generated to illustrate the probability to pass USP <905> for T = 96, 98, 100, 102 and 104, assuming the true batch mean is μ, and the true batch standard deviation is σ. These curves, shown in Fig. 24.1, were created by computer-generating 10,000 tier 1 data sets from a normal distribution with mean μ and standard deviation σ. The probability to pass tier 1 testing was estimated by taking the proportion of tier 1 data sets that passed the tier 1 criteria. For computer-generated data sets that failed tier 1 testing, additional tier 2 data were generated from the same normal distribution. The combined tier-1/tier-2 data were then evaluated through the tier 2 criteria. The total passing rate probability was estimated by calculating the proportion of data sets that passed either tier 1 or tier 2.
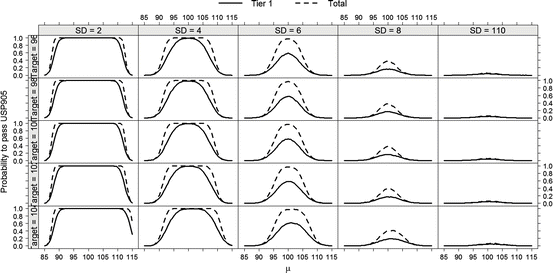

Fig. 24.1
OC curves for USP <905>, Rows display T (Target) values between 96 and 104
As seen in Fig. 24.1, for batches with smaller variability (σ = 2, 4), there is little increase in the probability that a batch will pass the criteria in USP <905> at tier 2 if it has failed to pass tier 1. For batches with larger standard deviation (e.g., σ = 6), tier 2 testing increases the probability of passing when the batch is on target. When the standard deviation is larger (e.g., σ = 8), there is little probability of passing USP <905> criteria even at tier 2. The graphs in Fig. 24.1 also demonstrate how use of the reference value (M) creates a zone of indifference to the mean. The zone of indifference, discussed in Lostritto (2012) establishes the same requirements on the size of the standard deviation whether the average of the dosage units is at target (100 %), or slightly off target (as low as 98.5 %, and as high as 101.5 %). Lostritto (2012) also discusses the potential use of an alternative test based on a parametric tolerance interval approach, discussed later in this chapter.
24.3 Process for Establishing Content Uniformity Using USP <601>: Aerosols, Nasal Sprays, Metered-Dose Inhalers, and Dry Powder Inhalers
USP <601> applies to aerosols, nasal sprays, metered dose-inhalers and dry powder inhalers. Specific tests for uniformity are provided for inhalation and nasal products that are not packaged in single unit dosage forms; these products have multiple doses within one unit. The USP test places requirements on doses collected at the beginning of unit life (sometimes called beginning of use) and end of unit life (or end of use). While the current version of USP <601> does not offer criteria to establish content uniformity, we discuss the method suggested in the 2011 proposed revision to the USP to illustrate the ‘zero tolerance’ approach to establishing content uniformity. As with USP <905>, this is a two tiered test. The test described in the suggested revisions to USP <601> is as follows.
Tier 1
Sample one beginning-of-use delivered dose, and one end-of-use delivered dose from each of ten containers for a total of twenty results, X 1B, X 2B, … X nB, n = 10 (beginning of use results) and X 1E, X 2E, … X nE, n = 10 (end of use results). These 20 results must meet the following criteria (tier 1).
1.
No more than 2 of the 20 results are outside of the range 80–120 % of label claim.
2.
No results are outside of the range of 75–125 % of label claim.
3.
The mean at each of beginning of use falls within the range of 85–115 % of label claim. In other words,
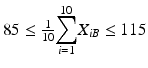 and
and 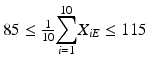 .
.If tier 1 criteria are not met, tier 2 criteria may be applied if:
1.
No more than 6 doses of the 20 doses collected are outside of 80–120 % of the label claim,
2.
None of the 20 results is outside of 75–125 % of label claim,
3.
The means for each of the beginning and end delivered doses fall within 85–115 % of label claim.
Tier 2 Criteria
Sample one beginning-of-use delivered dose, and one end-of-use delivered dose from each of an additional twenty containers and combine these results with those from tier 1 for a total of sixty results: X 1B, X 2B, … X nB, n = 30 (beginning of use results) and X 1E, X 2E, … X nE, n = 30 (end of use results). These results must meet the following criteria (tier 2):
1.
No more than 6 of the 60 results are outside of the range 80–120 % of label claim,
2.
No results are outside of the range of 75–125 % of label claim,
3.
The mean at each of beginning of use falls within the range of 85–115 % of label claim. In other words,
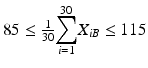 and
and 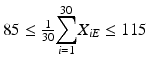 .
.Example
Ten metered dose inhalers are tested at beginning of use (BOU) and end of use (EOU); the results from canisters 1–10 are shown in Table 24.1. Applying tier 1 criteria, only two results are outside of the 80–120 % label claim, and none is outside of 75–125 %. The mean results for BOU and EOU are both within 85–115 %, so the requirements for establishing content uniformity per the suggested revisions to USP <601> are met.
Table 24.1
Example 2, metered dose inhaler
Canister | BOU | EOU |
|---|---|---|
1 | 109.6 | 93.7 |
2 | 78.8 | 110.1 |
3 | 75.6 | 116.8 |
4 | 103.7 | 116.5 |
5 | 102.6 | 112.1 |
6 | 86.1 | 104.5 |
7 | 102.6 | 108.6 |
8 | 101.3 | 92.4 |
9 | 102.8 | 105.7 |
10 | 104.7 | 95.5 |
Mean | 96.78 | 105.59 |
24.3.1 Discussion of Zero Tolerance Approach Described in the 2011 Suggested Revision USP <601>
Operating characteristic curves were generated for the suggested revisions to USP <601>, shown in Fig. 24.2. These curves were constructed by creating and subsequently testing 10,000 normally-distributed tier 1 data sets with mean μ and standard deviation σ in a fashion similar to that given in the USP <905> section, but using the USP <601> criteria. Additional tier 2 data were generated for failed tier 1 data sets that met criteria to move to tier 2. The tier 1 and total passing rate probability were estimated by calculating the proportion of data sets that respectively passed tier 1 testing and either tier 1 or tier 2. The OC curves demonstrate that tier 2 testing provides little or no benefit over tier 1 testing for virtually any batch mean and standard deviation. Because of this testing characteristic, there has been significant discussion around the use and properties of this test. An alternative test based on a parametric tolerance interval approach was proposed by the FDA (Nasr 2005) and will be discussed later in this chapter.
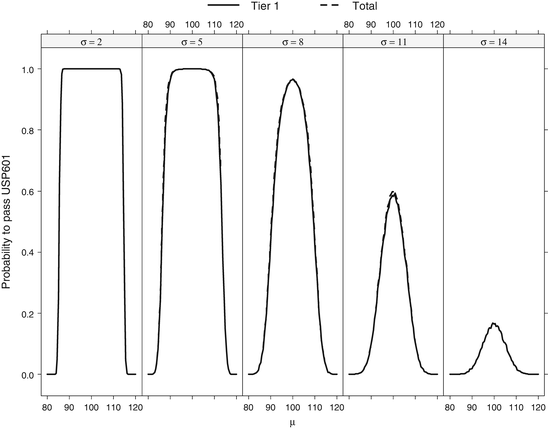

Fig. 24.2
OC curves for Revision Suggestions (2011) USP <601>
24.4 Process for Establishing Content Uniformity Using USP <3> Topical and Transdermal Drug Products
USP <3> may be used to assess uniformity in containers of multi-dose topical products. In contrast, USP <905> may be used to assess transdermal drug products and for dosage forms packed in single-unit containers. Topically applied drug products include, but are not restricted to creams, gels, ointments, pastes, suspensions, lotions, and foams. The uniformity of dosage units test described in USP <905> is appropriate for dosage forms packed in single-unit containers; however, as topically applied semi-solid drug products may experience physical separation during manufacturing processes and during their shelf life, the within-tube content uniformity should also be evaluated.
The procedure for evaluating within-tube content uniformity described in USP <3> depends on the size of the container. For multiple dose products that contain 5 g or more, there are two procedures that may be followed.
Procedure 1 (products 5 g or more)
1.
From a single tube, assay the active ingredient from an appropriate amount of product taken from the top, middle, and bottom portions of the tube for a total of three assay results: X 1, X 2, and X 3..
2.
Acceptance Criteria A are met if all assay results are within the product assay range and the relative standard deviation (RSD) is no more than (NMT) 6 % or as specified in the product specification or compendial monograph. The RSD is calculated as 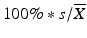 , where n = 3, and
, where n = 3, and
![$$ \overline{X}=\frac{1}{n}{\displaystyle \sum_{i=1}^n}{X}_i,\;s={\left[\frac{{\displaystyle {\sum}_{i=1}^n}{\left({X}_i-\overline{X}\right)}^2}{n-1}\right]}^{\frac{1}{2}}. $$](/wp-content/uploads/2016/07/A330233_1_En_24_Chapter_Equb.gif)
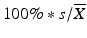 , where n = 3, and
, where n = 3, and![$$ \overline{X}=\frac{1}{n}{\displaystyle \sum_{i=1}^n}{X}_i,\;s={\left[\frac{{\displaystyle {\sum}_{i=1}^n}{\left({X}_i-\overline{X}\right)}^2}{n-1}\right]}^{\frac{1}{2}}. $$](/wp-content/uploads/2016/07/A330233_1_En_24_Chapter_Equb.gif)
3.
If the product fails Acceptance Criteria A, test three additional tubes from the same batch following step 1 described above and apply Acceptance Criteria B.
4.
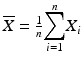 , n = 12,
, n = 12, ![$$ s={\left[\frac{1}{4}{\displaystyle \sum_{j=1}^4}Va{r}_{Tube\ j}\right]}^{\frac{1}{2}}, $$](/wp-content/uploads/2016/07/A330233_1_En_24_Chapter_IEq24.gif) < div class='tao-gold-member'>
< div class='tao-gold-member'>




Acceptance Criteria B are met if all of the results are within the product assay range and the RSD of the 12 assay results is NMT 6 % or as specified in the product specification or compendial monograph. In determining the RSD from multiple tubes, first determine the variance from the three measurements for each tube and average across the tubes. The RSD is calculated using this average variance so that 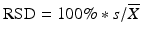 where
where
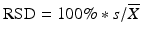 where
where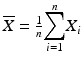 , n = 12,
, n = 12, ![$$ s={\left[\frac{1}{4}{\displaystyle \sum_{j=1}^4}Va{r}_{Tube\ j}\right]}^{\frac{1}{2}}, $$](/wp-content/uploads/2016/07/A330233_1_En_24_Chapter_IEq24.gif)
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


