Figure 10-1 Arrhythmia classification. AVB, atrioventricular block; AVNRT, atrioventricular nodal reentrant tachycardia; AVRT, atrioventricular reentrant tachycardia; bpm, beats per minute; SVT, supraventricular tachycardia; VT, ventricular tachycardia.
TACHYARRHYTHMIAS
- Tachyarrhythmias exhibit a heart rate >100 bpm and can arise from either supraventricular or ventricular origins.
- Although the appropriate management is dictated by the origin and the mechanism (increased automaticity, reentry, or triggered activity), these details are often not immediately evident.
- For practical purposes, a distinction is often made between narrow-complex and wide-complex tachycardias (WCTs) on the basis of a QRS complex < or >120 ms.
NARROW-COMPLEX TACHYCARDIA
- Narrow-complex tachyarrhythmias invariably originate above or at the atrioventricular (AV) node with a resultant narrow QRS complex that reflects normal activation along the His-Purkinje system.
- Narrow-complex tachycardias are further divided based on the regularity of the rhythm.
- These arrhythmias include sinus tachycardia, atrial fibrillation (AF), atrial flutter, atrial tachycardias, and various reentrant arrhythmias (e.g., atrioventricular reentrant tachycardia [AVRT], atrioventricular nodal reentrant tachycardia [AVNRT]).
Initial Approach
- The first priority is to check the patient’s vital signs and initiate the appropriate ACLS protocol if the patient is unstable.
- In the stable patient, obtain a 12-lead ECG. However, correct identification of the arrhythmia is often difficult with rapid heart rates.
- A helpful approach to tachyarrhythmias is to promote vagal (parasympathetic) activity with either carotid massage or administration of adenosine (Table 10-1). This serves to slow down AV nodal conduction, decreasing the rate and frequently allowing identification of the rhythm. In addition, reentrant rhythms that require the AV node for maintenance will be terminated by these maneuvers.
- Carotid sinus massage
- In the absence of carotid bruits, apply circular pressure to one carotid sinus for 5 seconds.
- Use caution in patients at risk for myocardial ischemia or cerebrovascular accident.
- In the absence of carotid bruits, apply circular pressure to one carotid sinus for 5 seconds.
- Adenosine (Table 10-1)
- Give 6-mg rapid IV push. Adenosine has a half-life of approximately 9 seconds, so the full dose must be pushed and flushed rapidly. A second dose of 12 mg can be used if the first dose has no effect.
- Adenosine will cause complete heart block, although typically brief; appropriate resuscitation equipment and personnel should be available.
- The patient should be warned that adenosine will cause a transient unpleasant feeling of presyncope.
- Rarely, adenosine can cause severe bronchospasm and severe respiratory distress.
- Give 6-mg rapid IV push. Adenosine has a half-life of approximately 9 seconds, so the full dose must be pushed and flushed rapidly. A second dose of 12 mg can be used if the first dose has no effect.
TABLE 10-1 Antiarrhythmic Medications
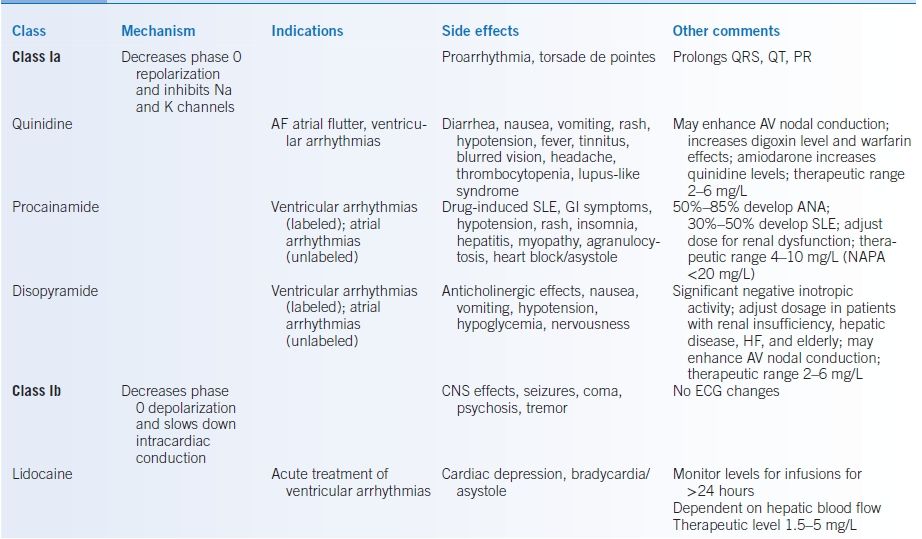
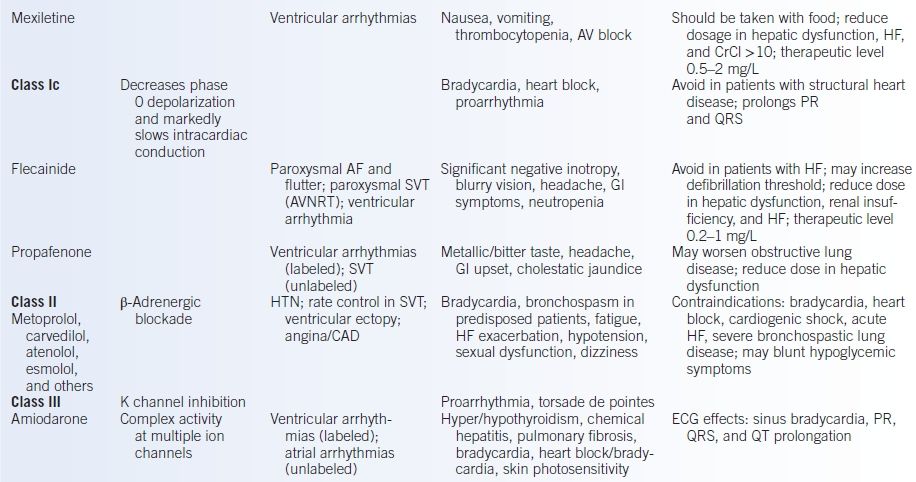
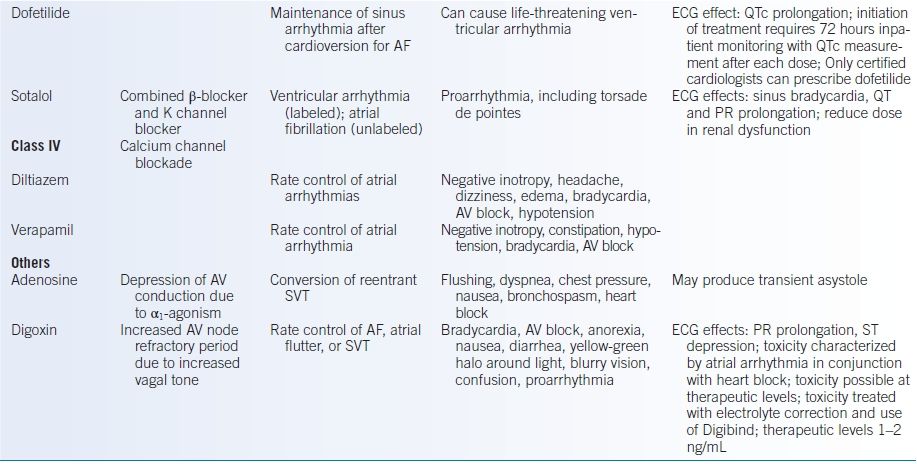
AF, atrial fibrillation; ANA, antinuclear antibody; AV, atrioventricular; AVB, atrioventricular block; AVNRT, atrioventricular nodal reentrant tachycardia; CAD, coronary artery disease; HF, heart failure; CNS, central nervous system; CrCl, creatinine clearance; GI, gastrointestinal; HTN, hypertension; K, potassium; NAPA, n-acetyl procainamide; SLE, systemic lupus erythematosus; SVT, supraventricular tachycardia.
Sinus Tachycardia
GENERAL PRINCIPLES
- Although not an arrhythmia per se, it is important to consider sinus tachycardia when confronted with a rapid, narrow-complex tachycardia.
- Sinus tachycardia is almost invariably a response to an underlying condition such as fever, hypovolemia, pain, anemia, thyrotoxicosis, pulmonary disease, heart failure (HF), caffeine, illicit drug use/withdrawal, or anxiety.1
- In rare cases, however, the sinus tachycardia is genuinely inappropriate and may be due to a reentrant arrhythmia within the sinoatrial (SA) node.2
DIAGNOSIS
- Heart rate is typically 150 to 200 bpm. There is usually a slow increase and decrease in heart rate, not abrupt onset and termination.
- ECG will demonstrate P waves with a normal axis and morphology.
- Laboratory evaluation should include underlying causes such as anemia, thyrotoxicosis, and drug intoxication (either prescribed or illicit).
TREATMENT
- Treatment is focused on the underlying cause.1
- Treatment aimed solely at slowing the heart rate (e.g., β-blockers or calcium channel blockers [CCBs]) is rarely appropriate and should be pursued only if the underlying cardiac disease (e.g., valvular disease, coronary artery disease [CAD], HF) results in intolerance of the elevated heart rate. The increased heart rate frequently represents a compensatory response, which is necessary to maintain cardiac output.
- It should be emphasized that an inappropriate sinus tachycardia is a rare condition and should be considered only after a rigorous exclusion of secondary causes of tachycardia.
Atrial Fibrillation
General Principles
- AF is the most common sustained arrhythmia with an incidence of about 1% in the general population and about 10% in those >80.1
- It is most commonly associated with valvular disease, advanced age, hypertension, HF, CAD, and mechanical dilatation of the atria.1,3
- Hyperthyroidism is the most common noncardiac, treatable cause of AF.
- Frequently described as “lone” (i.e., occurring in the absence of other cardiac diseases), “first episode,” “recurrent,” “paroxysmal” (i.e., recurrent episodes that typically self-terminate), “persistent” (i.e., requiring electrical or chemical cardioversion), and “permanent” (i.e., cannot be converted to normal sinus rhythm).
- AF results in three distinct consequences.
- The loss of AV synchrony with a resultant decrease in cardiac output due to the lack of the atrial contraction
- Increased thromboembolic risk due to blood stasis in the noncontractile atrium
- Decreased cardiac output and increased myocardial oxygen demand due to the increased ventricular rate
- The loss of AV synchrony with a resultant decrease in cardiac output due to the lack of the atrial contraction
- The underlying electrical substrate for AF remains under investigation, although the role of the pulmonary veins as a site of initiation has led to new treatment options (see Treatment section below).
Diagnosis
- ECG demonstrates chaotic atrial activity without evidence of P waves, although some coarse fibrillation waves may be evident in coarse AF (Fig. 10-2).
- Ventricular rate is typically 140 to 180 bpm but varies substantially depending on rapidity of AV nodal conduction.
- Laboratory evaluation should include tests of thyroid function.
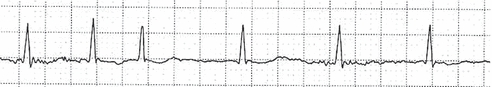
Figure 10-2 Atrial fibrillation. Note the irregular rhythm and lack of P waves.
Treatment
- Despite the intuitive appeal of treatment, which maintains sinus rhythm, several studies have shown that this strategy is unsuccessful at reducing the morbidity and mortality associated with AF, most likely reflecting the poor success rate of current therapies at maintaining sinus rhythm.4–6 Therefore, current therapy focuses on addressing individually the adverse consequences of AF.
- The two general treatment strategies are often referred to as “rate control,” in which the AF rhythm is accepted, but the rate is controlled and “rhythm control,” in which efforts are made to maintain sinus rhythm. As discussed below, both strategies frequently require anticoagulation.6
Control of Ventricular Rate
- Ventricular rate is controlled with medications that slow down AV nodal conduction.
- β-Blockers and CCBs are first-line agents for control of ventricular rate. Choice of specific agents is often determined by other indications or contraindications in a given patient.
- Digoxin can also provide control of resting ventricular rate, although it is less effective at reducing ventricular rate during exertion. In addition, digoxin is associated with more side effects than are other agents (Table 10-1). Its ideal use is in a patient with left ventricular (LV) dysfunction whose ventricular function also benefits from digoxin.
- In some cases, pharmacologic control of ventricular rate proves impossible. In these patients, invasive radio-frequency AV node ablation (resulting in complete heart block) with implantation of a permanent pacemaker is an option.
Maintenance of Sinus Rhythm
- Restoration and maintenance of sinus rhythm is a tempting goal but not always readily achievable. How aggressively sinus rhythm is pursued is dictated by the patient’s overall cardiac function and the degree to which they can tolerate AF.
- Initial termination of AF is accomplished by synchronized direct current cardioversion (DCCV) with a success rate of ≥80%. DCCV requires sedation and hemodynamic monitoring and should be carried out in facility with emergency resuscitation and airway support available.
- Several medications may result in pharmacologic cardioversion, increase the success rate of DCCV, and improve maintenance of sinus rhythm once cardioversion is accomplished.1
- For patients with structural heart disease, preferred agents include amiodarone, sotalol, and dofetilide (Table 10-1).
- For patients without structural heart disease, preferred agents include amiodarone, flecainide, and propafenone (Table 10-1).
- Amiodarone (Table 10-1) is typically well tolerated but has several significant long-term side effects. It is, therefore, less favored for use in young patients who may require decades of therapy.7
- Initiation or adjustment of these medications frequently requires inpatient cardiac monitoring and should be performed in consultation with a cardiac electrophysiologist.
Atrial Fibrillation Ablation/Surgical Treatment Options
- New techniques of catheter-based AF ablation are becoming increasingly successful.
- Should noninvasive attempts to maintain sinus rhythm fail in a patient who does not tolerate AF well, invasive ablation techniques can be considered to restore the sinus rhythm.6
- Historically, the surgical Cox maze procedure was designed to eliminate AF by creating a pattern of scar lines in the atrium that interrupts the fibrillation. However, this technique is now more commonly performed in conjunction with other cardiac operations.
- Minimally invasive catheter-based techniques (such as electrical isolation of the pulmonary veins) may be a more suitable option for patients not requiring cardiac surgery with a success rate of 60% to 80% at experienced centers.
- The details of these techniques continue to undergo rapid development and are beyond the scope of this chapter.
- Consultation with a cardiac electrophysiologist is warranted for any patient with poorly tolerated AF.
Thromboembolic Risk
- Although, in theory, restoration of sinus rhythm obviates the need for anticoagulation, several studies have shown that the risk of stroke from atrial thrombi is essentially unchanged by pharmacologic attempts to maintain sinus rhythm.4,5 However, given the risks of anticoagulation, attempts have been made to identify which patients are at high enough risk to justify warfarin therapy.
- Several different indices of thromboembolic risk have been developed. In general, patients with advanced age, HF, a history of stroke, diabetes, or hypertension are at greater risk of stroke in the context of AF.4
- It is essential to ensure the absence of left atrial thrombus prior to DCCV in any patient with AF lasting longer than 48 hours. This can be accomplished with transesophageal echocardiography with visualization of the left atrial appendage; transthoracic echocardiography is not adequate. Alternatively, the patient can be anticoagulated for a period of at least 3 to 4 weeks prior to cardioversion.4
- Importantly, the risk of embolic events is highest in the several weeks following cardioversion, even if it is successful. It is therefore essential to continue therapeutic anticoagulation for at least 4 weeks following cardioversion.
- In recent years, a number of new agents have been approved for thromboembolism prevention in nonvalvular AF. The choice of a particular therapeutic anticoagulant should be tailored to each patient individually (see Chapter 14).
- The role of transesophageal echocardiogram (TEE) to exclude thrombus prior to DCCV when therapeutically anticoagulated with an agent other than warfarin has not been well established.
- In recent years, a number of new agents have been approved for thromboembolism prevention in nonvalvular AF. The choice of a particular therapeutic anticoagulant should be tailored to each patient individually (see Chapter 14).
- It should be emphasized that AF is typically a chronic/recurrent condition. The presumption is, therefore, that the patient requires permanent anticoagulation unless (a) a contraindication to anticoagulation exists or (b) the patient is clearly at low risk for embolic events.
AF and Preexcitation (Wolff-Parkinson-White Syndrome)
- AF in a patient with an AV bypass tract poses a special risk. In the normal heart, the maximal ventricular rate in AF is limited by the slow conduction of the AV node. When conduction occurs through a bypass tract, the ventricular rate can match the AF rate (400 to 700 bpm) resulting in degeneration into ventricular fibrillation (VF) and cardiovascular collapse.
- The ECG in preexcited AF is characterized by an irregularly irregular rate with varying morphologies of a wide-complex QRS.
- Treatment options include procainamide, amiodarone, or DCCV. AV nodal blocking agents including adenosine, CCBs, β-blockers, and digoxin should be avoided as they can cause acceleration of the bypass tract conduction. Expert consultation is required for definitive treatment and ablation of the bypass tract (Table 10-1).
Perioperative AF
- AF is common in the postoperative patient, especially after cardiac surgery and most specifically valvular surgery. Most episodes are self-limiting but in the interim pose the same risks as any other episode of AF. Because of the potential complications of anticoagulation, prompt DCCV within the first 48 hours of AF is recommended.
- Perioperative treatment with β-blockers has been shown to reduce the incidence of AF.8
- In addition, amiodarone (Table 10-1) is frequently used as either prophylactic treatment or once the AF has occurred.8
- The need for continued therapy should be reevaluated by a cardiologist several months after the operation.
Atrial Flutter
GENERAL PRINCIPLES
- Atrial flutter is sustained reentry within the atria, causing rapid fluttering of the atria.
- Although it is more electrically organized than AF, the atrial transport of blood is still less efficient than normal; flutter thus also has a risk of thromboembolism.
- Many of the same factors that predispose to AF are also related to atrial flutter. It is not uncommon for patients to have both rhythms and transition from one to the other.
DIAGNOSIS
- ECG demonstrates sawtooth flutter waves. In typical flutter, these sawtooth waves are most evident in the inferior leads (Fig. 10-3). Typical flutter waves have a negative vector in the inferior leads (II, III, aVF) while positive in the anterior precordium (V1, V2). In other forms of flutter, various appearances of the flutter waves are possible.
- Flutter waves are typically at a rate of 240 to 340 bpm; 300 bpm is classic.
- Ventricular rate is usually at a 2:1, 3:1, or 4:1 ratio with the atrial rate. Although the ventricular rate may be irregular due to variable conduction block, it is more typically regular with a fixed ratio to the atrial activity.
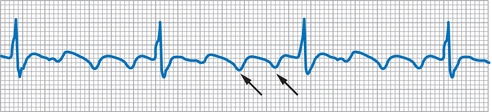
Figure 10-3 Atrial flutter. This is an example of 3:1 atrial flutter. Note sawtooth-shaped flutter waves (arrows).
TREATMENT
- Medical treatment of atrial flutter is essentially identical to management of AF: control of ventricular rate, management of stroke risk with anticoagulation, and, when possible, maintenance of sinus rhythm.
- The stereotypical circuit in typical atrial flutter is readily amenable to catheter ablation techniques with a success rate of approximately 90%. However, a significant number of patients subsequently develop AF.6
Reentrant Supraventricular Tachycardia
GENERAL PRINCIPLES
- Although the term supraventricular tachycardia (SVT) would technically include all rhythms arising above the AV node, it is conventionally applied to a specific group of reentrant rhythms.9,10
- SVTs are divided into two main groups based on the anatomy of the reentrant circuit.
- AVNRT: The entire reentrant circuit is contained in the AV node and the immediate surrounding tissue.
- AVRT: The reentrant circuit includes atrial tissue, the AV node, ventricular tissue, and an accessory bypass tract.
- AVNRT: The entire reentrant circuit is contained in the AV node and the immediate surrounding tissue.
- Common features of reentrant rhythms include one branch of the circuit with rapid conduction (and a long refractory period) and another branch with slow conduction (and a short refractory period). With this anatomy, an appropriately timed premature impulse can trigger the reentry and result in continuous cycling of electrical activity in this circuit.
- Different forms of AVRT and AVNRT are characterized by whether the antegrade impulse (forward, i.e., atrial to ventricular) occurs over the fast or slow pathway. The retrograde impulse (backward, i.e., ventricular to atrial) occurs over the other pathway.
- SVTs result in retrograde P waves as the retrograde signal stimulates the atrium. These rhythms can therefore be further divided into long RP in which the (retrograde) P wave occurs significantly after the QRS complex, reflecting retrograde conduction over a slow pathway, or short RP in which the (retrograde) P wave occurs rapidly after the QRS.
- AVRT and AVNRT typically occur in patients without other underlying cardiac diseases.
DIAGNOSIS
- Clinical presentations include palpitations, dyspnea, syncope, and angina/HF in patients with underlying cardiac disease.
- Accurate diagnosis of reentrant arrhythmias is frequently possible from the ECG.
- Reentrant rhythms typically have an abrupt onset and termination, in contrast to sinus tachycardia and AF, and exhibit heart rates in the range of 150 to 250 bpm.
- All reentrant SVTs exhibit retrograde P waves, which are negative in leads II, III, and aVF, reflecting activation of the atrium in a reverse, caudal-to-cranial direction.
- AVNRT represents approximately 70% of SVTs with AVRT constituting the remainder.1
- ECG features of common SVTs are summarized in Figure 10-4.
- Typical AVNRT (50% to 90% of AVNRTs): Antegrade conduction occurs over the slow pathway and retrograde conduction over the fast pathway. As a result, the retrograde P wave occurs within 100 ms of the QRS, making this a “short RP” rhythm. In fact, the RP interval is so brief that typically the P waves are obscured by the QRS or visible only as a pseudo-R in lead V1 or pseudo-S in lead II or III.
- Atypical AVNRT: Antegrade conduction occurs over the fast pathway and retrograde conduction over the slow pathway. As a result, the retrograde P wave is visible between the QRS complexes with a long RP interval.
- Orthodromic AVRT (95% of AVRTs): Antegrade conduction occurs via the AV node and retrograde conduction over the accessory pathway. Most commonly, the RP interval is short due to a fairly rapidly conducting accessory tract. However, a slowly conducting accessory tract is also possible and gives rise to a long RP tachycardia.
- Antidromic AVRT: This is a WCT but mentioned here for contrast with orthodromic AVRT. Antegrade conduction occurs over the accessory pathway with a resultant wide QRS complex.
- AVNRT may be visualized as reentry within the AV node. As a result, the tachycardia can continue regardless of events in the atria or ventricles, such as premature ventricular contractions (PVCs), premature atrial contractions (PACs), or bundle-branch block. In AVRT, the atrium and ventricle are part of the circuit. Atrial and ventricular events will therefore affect the tachycardia.
- AVRT requires the existence of an accessory tract. Accessory pathways may be “manifest,” that is, visible as preexcited delta waves on the baseline ECG (Wolff-Parkinson-White pattern), or concealed, that is, lacking evidence of antegrade conduction but capable of retrograde conduction and therefore support of AVRT. A manifest pathway can result in either orthodromic or antidromic AVRT, whereas a concealed pathway is capable only of orthodromic AVRT.
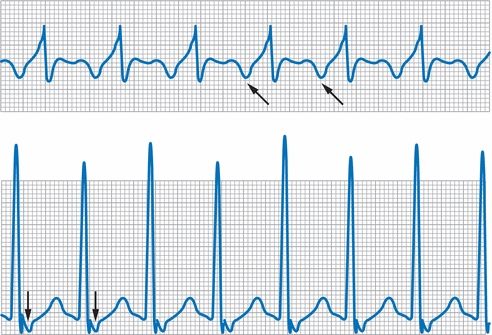
Figure 10-4 Supraventricular tachycardia. Above, SVT with a long RP interval; note inverted retrograde P waves in lead II (arrows). Below, SVT with a short RP interval; note inverted retrograde P waves in lead II (arrows).
TREATMENT
- The initial approach to a stable patient with a narrow-complex tachycardia is the use of vagal maneuvers such as the Valsalva maneuver or carotid sinus massage or the administration of adenosine, as discussed above (Table 10-1). This will usually result in the termination of the arrhythmia in the case of AVRT and AVNRT.
- Medical treatment consists of AV node blockade with β-blockers, CCBs, and digoxin (Table 10-1).
- DCCV usually terminates the arrhythmia and is required in unstable patients.
- The high success rate (>95%) of catheter ablation makes definitive invasive treatment of these rhythms equally first line with medical management.9
Atrial Tachycardia
- Atrial tachycardias encompass various intra-atrial arrhythmias, including intra-atrial reentry, automatic tachycardias, and triggered tachycardias.
- These rhythms are almost invariably associated with underlying cardiac disease, chronic obstructive pulmonary disease (COPD), electrolyte imbalances, or digoxin toxicity (Table 10-1).1
- ECG features of atrial tachycardia
- P-wave axis and morphology are different from sinus rhythm.
- Rhythm is typically regular.
- QRS is usually identical to normal sinus rhythm.
- An electrophysiology (EP) study is usually required to fully characterize the atrial arrhythmia.
- P-wave axis and morphology are different from sinus rhythm.
- Medical treatment options are focused on treatment of the underlying abnormalities.
- For clinically significant atrial tachycardias, radiofrequency catheter ablation is often the treatment of choice.
Multifocal Atrial Tachycardia
- Multifocal atrial tachycardia (MAT) is almost invariably associated with COPD or HF.11
- The underlying electrophysiologic mechanism likely involves increased automaticity or triggered activity.
- ECG features of MAT
- Three or more different P-wave morphologies associated with different PR intervals (Fig. 10-5)
- Atrial rate typically 100 to 130 bpm
- Three or more different P-wave morphologies associated with different PR intervals (Fig. 10-5)
- Treatment is focused on the underlying disease with little role for antiarrhythmic medications.
- In cases where treatment is necessary, CCBs and amiodarone (Table 10-1) have been shown to have some success.
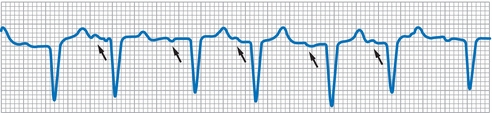
Figure 10-5 Multifocal atrial tachycardia. Note irregular rhythm and P wave with varying morphology and PR interval (arrows).
WIDE-COMPLEX TACHYCARDIAS
- A WCT reflects activation that proceeds through the myocardium without use of the His-Purkinje system or does so in a slow and disorganized manner.
- As a result, the QRS is wide due to the slow propagation of the electrical signal. Only a few rhythms generate a wide QRS tachycardia:
- A ventricular rhythm, that is, ventricular tachycardia (VT)
- A supraventricular rhythm with aberrancy, that is, an intraventricular conduction delay such as left bundle branch block (LBBB) or right bundle branch block (RBBB)
- A supraventricular rhythm with excitation through an accessory tract
- Metabolic derangements (hyperkalemia or drug toxicity) resulting in a wide QRS
- A ventricular rhythm, that is, ventricular tachycardia (VT)
Initial Approach
- As with narrow-complex tachycardias, the first priority is evaluation of the patient’s stability and application of ACLS protocols to the unstable patient.
- The immediate question that must be addressed when confronted with a WCT is whether the rhythm is ventricular in origin or supraventricular with aberrancy or preexcitation.
- Attention must also be paid to possible metabolic causes of a wide QRS complex, most importantly drug toxicity and hyperkalemia.
- The distinction between VT and SVT with aberrancy is challenging and is not always readily possible. However, several algorithms have been developed to distinguish these entities.12
Suggestive Features of Ventricular Tachycardia
- There are several suggestive features of VT.
- An extreme rightward axis (−90 to 180 degree) suggests VT.
- An initial R wave in aVR suggests VT.
- Slight irregularity or irregularity at the onset of the rhythm suggests VT.
- A QRS >140 ms in an RBBB-like tachycardia or a QRS >160 ms in an LBBB-like tachycardia suggests VT.
- The presence of precordial concordance, that is, monomorphic QRS complexes, across the precordial leads that are either all entirely positive or all entirely negative suggests VT.
- The presence of fusion beats (combination of a normal QRS and the ectopic beat) and capture beats (intermittent normal QRS complexes within the tachycardia) indicates AV dissociation and thus indicates VT (Fig. 10-6).
- An extreme rightward axis (−90 to 180 degree) suggests VT.
- In addition, several stepwise algorithms have been developed to distinguish VT from SVT in a WCT. The most commonly used criteria are those published by Brugada et al. and are summarized in Table 10-2.13 At each step, either the rhythm is identified as VT or one proceeds to the next criterion.
- Although accurate, these criteria are frequently too cumbersome to be applied by those not readily familiar with them. The original Brugada criteria demonstrated a sensitivity of 99% and a specificity of 96.5%; however, further real-world studies demonstrated a sensitivity of 79% to 92% and a specificity of 44% to 56%.14
- A useful rule of thumb is that any WCT is presumed to be VT until proven otherwise. This assumption is justified by the fact that up to 80% of WCT in the setting of heart disease is VT.
- This approach is further reinforced by the fact that many of the pharmacologic treatments for SVT (adenosine and CCBs) have the potential to cause degeneration of VT to VF, whereas the treatments for VT (amiodarone and procainamide) are frequently effective and safe for SVT (Table 10-1). Therefore, use of VT treatments is preferred if the rhythm is unclear.
- In summary, any WCT should be managed as VT. Once the patient is stabilized, expert consultation is warranted to clarify the rhythm and future management.
TABLE 10-2 Brugada Criteria for Wide-Complex Tachycardia

AV, atrioventricular; VT, ventricular tachycardia; LBBB, left bundle-branch block; RBBB, right bundle-branch block.
Modified from Brugada P, Brugada J, Mont L, et al. A new approach to the differential diagnosis of a regular tachycardia with a wide QRS complex. Circulation 1991;83:1649–1659.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


