FIGURE 37-1A AND 37-1B PA (A) and lateral (B) chest radiograph demonstrating elevated right hemidiaphragm due to phrenic nerve injury following cryoablation.
EXPERT OPINION
This case highlights the risk of phrenic nerve palsy associated with cryoballoon ablation and the importance of early recognition of injury to the phrenic nerve. The course of the phrenic nerve typically traverses anterior to the right-sided pulmonary veins and may be injured during cryoapplication, particularly in the RSPV. A pacing catheter is usually inserted in a superior part of the superior vena cava to pace the phrenic nerve continuously during ablation. The simplest method of determining phrenic nerve capture is direct palpation of the abdomen to confirm contraction of the right hemidiaphragm. Phrenic injury during cryoapplication is often first recognized by reduced strength of diaphragmatic capture. Many other methods to determine phrenic capture have been developed, including direct measurement of diaphragmatic potentials via surface electrodes, and other means to detect the contraction, such as visualization with intracardiac ultrasound, auditory surveillance with Doppler ultrasound, and others. The earlier phrenic nerve injury is detected, the faster the recovery of diaphragmatic function, which often occurs by the end of the procedure.
Despite taking all these precautions, phrenic nerve paralysis will still occur, and often the patient is very symptomatic, with a recovery period of up to a year or more.
INTRODUCTION
The mainstay of an ablative approach to paroxysmal AF is pulmonary vein isolation using a single-tip radiofrequency catheter. In an attempt to eliminate the need for point-by-point ablation, balloon-based ablation systems have been developed to produce circumferential lesions.
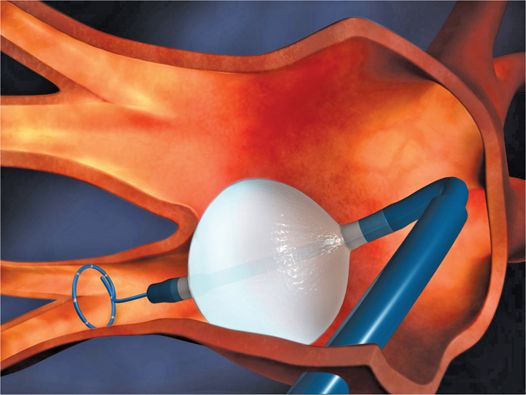
The most commonly used system is the Medtronic Arctic Front over-the-wire cryoballoon catheter. The catheter is introduced into the left atrium through a 12-Fr steerable sheath. The shaft of the cryoballoon catheter has a central lumen that can accommodate a wire or allow the passage of a spiral catheter for real time mapping and is used for injection of saline or contrast (Figure 37-2).
FIGURE 37-2 Illustration of the Medtronic Arctic Front cryoballoon catheter within a steerable sheath, with the Achieve spiral mapping catheter within the central lumen of the catheter. (Reproduced with permission of Medtronic, Inc.)
The inner balloon of the cryoballoon catheter is cooled to a temperature of −112°F (−80°C) with nitrous oxide.1 The cold outer skin of the balloon adheres to the underlying tissue (much like a tongue on a frozen metal pole), and a large thermal gradient removes heat causing irreversible cellular injury below 23°F (0°C). Cryothermal energy produces progressive necrosis but does not result in significant alteration of tissue structure at thaw.2 The cryothermal lesion formation can be divided in three sequential stages: the freeze/thaw phase, the hemorrhagic inflammatory phase, and the replacement fibrosis phase stage.2 Theoretically, the absence of endothelial disruption with cryoablation is thought to result in less thrombogenicity.2
PATIENT SELECTION
• Cryoablation is reserved for patients with drug-refractory symptomatic paroxysmal AF for which pulmonary vein isolation is the mainstay of therapy. It is not ideal for the patient with persistent or chronic AF where additional lesion sets are generally required, since additional ablation catheters are often necessary.
• We strongly recommend the use of imaging prior to the procedure with either a CT pulmonary venogram or an MRI to assess the suitability of the pulmonary venous anatomy for the balloon and to assist with sizing of the cryoballoon.
ADVANTAGES
• Shorter time to proficiency than point-by-point radiofrequency catheter ablation.
• Less dependent on operator dexterity.
• Potentially shorter procedural and fluoroscopy times in nonrandomized trials.3
DISADVANTAGES
• Not cost-effective if additional ablation is required to treat non-PV sources of arrhythmia, as is commonly encountered in patients with persistent AF, or if PV isolation cannot be achieved a high percentage of the time with cryoablation.
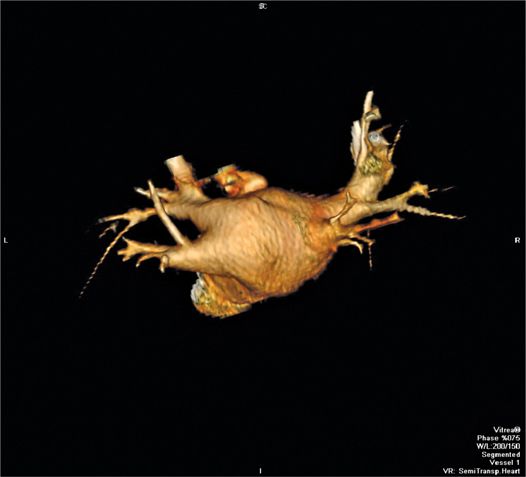
• Certain venous anatomy less compatible with cryoballoon ablation such as a large common left pulmonary vein trunk (Figure 37-3).
FIGURE 37-3 CT venogram of the left atrium showing a large common left pulmonary vein trunk, which is usually not suitable for cryoablation.
• Higher incidence of phrenic nerve paralysis.4
KEY PROCEDURAL STEPS
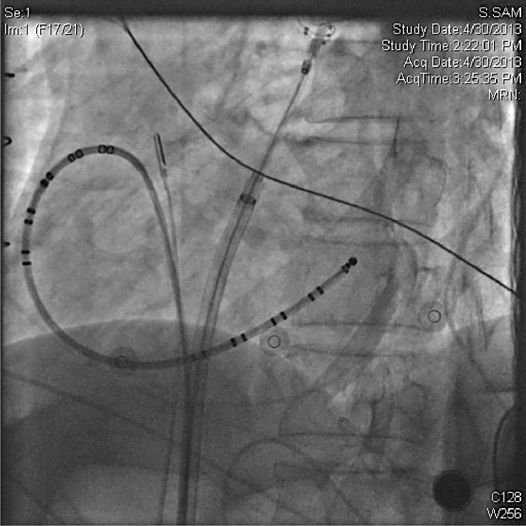
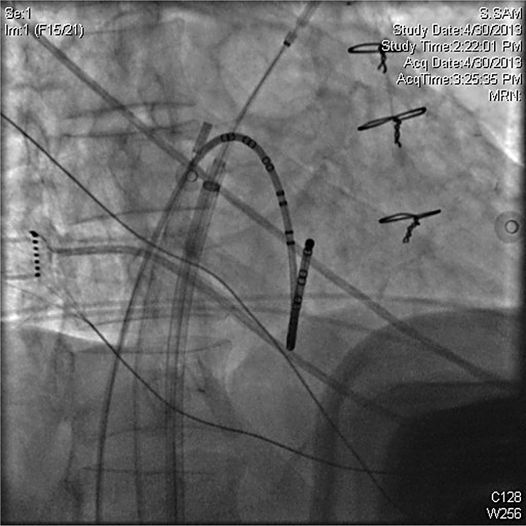
• Access: Given that most patients do not interrupt anticoagulation for the PVI procedure, safe vascular access is essential. We recommend micropuncture kits (21 gauge needle and 4 French dilator) for initial venous access prior to exchanging for larger sheaths. A minimum of two venous sheaths are required; one is exchanged for the steerable 12 Fr sheath after transseptal puncture and another as a conduit for a catheter to pace the phrenic nerve, which can be as small as 4 Fr. In our lab, we typically place a third venous sheath for the placement of a coronary sinus catheter and a fourth for placement of an intracardiac echocardiogram (ICE) probe (Figures 37-4 and 37-5).
FIGURE 37-4 LAO radiograph of standard set up: coronary sinus catheter, ablation catheter in SVC for phrenic nerve pacing, cryoballoon at antrum of left upper pulmonary vein, ICE catheter imaging from the right atrium at the level of the fossa.
FIGURE 37-5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree