Patient identification
Patient history
Specimen loss
Specimen identification
Specimen adequacy
Specimen handling
Specimen transportation
Accessioning data misentry
Many of the errors in the preanalytic phase occur before the specimen ever reaches the pathology lab. To avoid these types or errors, the LIS has to be ideally closely linked to the hospital system’s EMR. This permits electronic computerized provider order entry (CPOE) to take place. In addition, employing positive patient identification technologies (e.g., barcoded patient wristbands and biometrics) at the specimen submitting sites can help to thwart some of these errors from the outset [3, 17, 18].
Patient Identification
Positive patient identification technologies have had an impact on error reduction in medicine. Such technology helps to reduce errors even before specimens arrive in pathology laboratories. One of the barriers in using the same patient identification system for the EMR and LIS is the lack of standardized barcodes . Hence, barcodes used for patient identification are rarely compatible with those required by the LIS or middleware laboratory tracking system. This results in diverse patient identification data, and not surprisingly is a common source of preanalytical errors. This problem has been the focus of much effort in recent years, which has decreased identification error rates [19].
Positive patient identification technologies that are independent of printing include radiofrequency identification (RFID) tags [20]. RFID is becoming more affordable and accordingly being leveraged to help solve problems encountered with the use of printed barcodes [3, 18]. Another failure in the patient identification process is related to patient demographic data not being available when needed (e.g., at accessioning) , as occurs when the Admission-Discharge-Transfer (ADT) feed from the Hospital Information System (HIS) to the LIS is down. Most EMRs are built atop client-server architecture with routinely scheduled downtime for backup and maintenance. In addition, there are unscheduled and unexpected downtimes. Modern EMRs are evolving toward more evenly distributed architectures to minimize scheduled downtimes [21].
Lack of standards in barcode technology and labels may be an important contributor to preanalytic errors. For instance, there may be some overlap of symbologies used at the patient registration point and in the lab, which would present a problem if the barcode scanners used in the lab were able to scan either type of barcode. Standardization of barcodes in AP is important, and needs to be addressed, similar to what has been achieved in transfusion medicine and blood banking [3, 18, 22].
Many of preanalytical errors can also be traced to manual steps and processes, especially when these are not standardized and the lab utilizes workarounds for special instances. In general, a process that is more lean and that involves verification at each step will be less error-prone. Each step in the specimen life cycle should be part of a standard workflow process, and ideally follow that of an industrial workflow, with minimal involvement of humans and more emphasis on automation. The resulting Lean processes will help with error reduction [12, 14]. Table 5.2 lists the types of errors that may occur in the patient identification process.
Table 5.2
Potential sources of error in the patient identification process
The identification medium deteriorates (e.g., damaged barcode labels) resulting in “read-type” errors or failure to read |
Use of technology which may not be compatible with downstream systems (e.g., RFID) |
Patient data are not accessible from an offline or incompatible system |
Lack of standardized barcodes resulting in either no scans or errors in scanning of barcodes |
Incorrect patient identification data from an unverified source |
Manual entry errors |
Wrong wristband printed and applied to wrong patient |
Patient History
A major source of error in the preanalytical phase is a lack of relevant clinical history or incorrect clinical history on paper or electronic requisitions[23]. A large number of requisitions received by the AP lab often have inadequate or no clinical history on the accompanying requisition [24]. There is no question that appropriate and accurately provided clinical history can provide the AP lab with important background information about the specimen type, and further guides the appropriate triage of the specimen. For example, a cancer specimen will receive appropriate grossing and perhaps taking specific sections (e.g., tumor margin) versus a non-neoplastic specimen with inadequate clinical history. One of the strategies employed by AP labs to improve the adequacy of clinical history supplied on their requisitions is to reject requisitions with no or illegible clinical history [23, 24].
Another strategy the laboratory may employ to obtain clinical history is to directly access this data from the EMR or other information systems, to electronically extract any pertinent information from patient charts. Some EMRs can directly transmit this information into the LIS via an electronic interface [22]. This electronic dump of information may not always contain data that is relevant to the specimen and pathological evaluation that is being performed. Accordingly, it becomes more time consuming in such cases to sift through this superfluous information. Advances in technology are still required to ensure that AP labs are able to routinely receive relevant information for all cases as part of a standardized workflow [22].
In summary, in the preanalytic phase, an ideal state would be achieved when the right specimen is taken from the right patient, an event that is typically beyond the control of AP lab staff. This would be followed by traceable steps in which this specimen is properly identified, labeled, handled, and transported in a timely manner to the AP lab. The AP lab, upon receipt of this sample, needs to accession the specimen without errors [21, 24]. Table 5.3 lists multiple potential points of error that may occur during the accessioning step.
Table 5.3
Potential points of error during the accessioning process
Illegible handwriting |
Keying errors |
Transposing numbers/letters |
Wrong blocks printed and matched with wrong case |
Excessive time spent for manual verification |
Lack of a standardized workflow and reliance on a batch process |
Lack of relevant clinical information on the requisition |
Computerized Provider Order Entry
Many of the orders received by a clinical pathology laboratory information system (CPLIS) are handled by a CPOE system/interface [22]. However, in the APLIS, a similar CPOE system is not widely used. In many APLIS systems, the order entry is predominantly manual with the majority of “orders” supplied on paper requisitions. There are many advantages of CPOE, most notably the potential to diminish order-related errors. CPOE implementation for surgical pathology has unique challenges, mainly because surgical pathology orders need additional information when compared with clinical pathology (CP) orders. For example, ordering a serum PSA test only requires selecting this lab test in the electronic order set. By comparison, a surgical pathology order is more complex as the order also requires additional information to be relayed to the pathology lab such as anatomical location, organ/tissue type, relevant clinical information , and often pertinent information about the procedure [22]. In addition, a single order may include several parts from the same organ (e.g., margins versus tumor) or multiple parts from different organs. Table 5.4 lists some of the benefits of using a CPOE in the AP laboratory .
Table 5.4
Key advantages of using computerized provider order entry (CPOE) in the AP laboratory
Reduced turnaround time |
Reduced manual steps, including transcription, label writing, and accessioning |
Elimination of ambiguous/indecipherable orders |
Improved compliance with laboratory testing and/or clinical guidelines |
Improved test utilization and appropriateness of test ordering |
Direct feed of patient clinical information (e.g., history, problem list, etc.) into the APLIS (i.e., reverse flow of data from the EMR) |
Pathology Asset Tracking
As mentioned, laboratory misidentification errors may be preanalytic or due to postanalytic errors in the test cycle [3, 24]. Asset tracking solutions in pathology continue to evolve, and provide labs with a scalable solution for quality management and error reduction [22, 26–29]. Misidentification errors in the AP laboratory may result in an adverse event causing unnecessary subsequent procedures or even death. [25] A Q-Probes study from the College of American Pathologists (CAP) involving 136 institutions provided information on a total of 1811 mislabeling occurrences, showing that overall mislabeling rates in participating labs were 1.1 per 1000 cases. Interestingly, 21 % of these errors occurred before accessioning, 12 % at accessioning, 22 % at block labeling, 10 % during gross pathology, and 30 % in histology [31]. As pathology laboratories become more subspecialized, coupling asset tracking technologies with Lean processing methods has the potential to reduce these errors, drive the workflow, and simultaneously make the process more efficient [18, 29]. Table 5.5 lists some of the advantages of barcoding and tracking solutions.
Table 5.5
Advantages of barcoding and tracking
Asset management (identification and tracking) |
Data input into the LIS is immediate and reliable: lower potential for data-entry errors than key entry |
Standardized workflow processes: |
Supports lab automation |
Just in time printing (e.g., labels) |
Promotes Lean processing and patient safety |
Improves overall turnaround time |
Drives the workflow process |
In the pathology lab, tracking begins with adding an identifier to each asset that needs to be tracked. Some examples of assets in the laboratory include specimen requisitions, patient specimens, and their derivatives such as tissue/cell blocks and glass slides (Fig. 5.1). Tracking of machine-readable identifiers such as barcodes and RFID tags has the ability to rapidly and accurately record the asset’s identifier into a tracking system (e.g., LIS and middleware [34]). The types of barcodes that can be used can be of either linear (1D) or matrix (2D) type. Linear bar codes have bars and spaces and can be either numeric (e.g., UPC) or alphanumeric (e.g., Code 128). Matrix (2D) barcodes (Fig. 5.1) have the following advantages [18]:
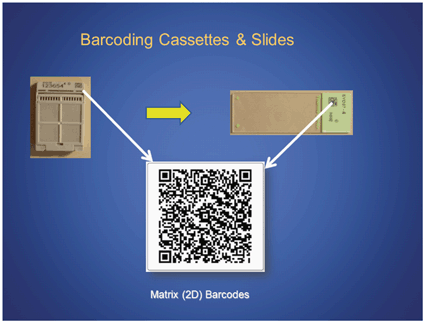

Fig. 5.1
Example of a matrix (2D) barcode and its application in assets (tissue blocks and slides) in the anatomical pathology (AP) laboratory
Higher data density (more characters and scalable)
Smaller barcodes, which is better for smaller labels
Allows omnidirectional scanning
Associated with less scan failures
Higher tolerance for printer failure/damage
Tracking systems have a variety of applications and capabilities ranging from identification of the location of the assets in the lab (or storage area) to real-time and dynamic monitoring of their status (i.e., what phase they are in of the workflow process) [18, 29]. This allows a tracking solution to be designed to help in controlling the speed of specimen flow within the lab, as well as in controlling various parts of the workflow of the laboratory. It also allows for collection of QA data to monitor such quality indicators as turnaround time. The functionality of the tracking system dictates what system requirements are needed (e.g., complex interfaces) and what type of an investment in infrastructure will be required (e.g., wired versus wireless networking). As technology matures, so too will the complexity and functionality of these asset tracking systems (Table 5.6) [18, 26].
Function | Tasks |
|---|---|
Auditing | Track asset events (what, when, and who) |
Audit trailing (when was asset last seen) | |
QA indicators and analysis of workflow | |
Creating dashboards | |
Workflow control | Prevents batch workflow |
Creates locks/gates on steps (e.g., if the right asset is not in association with the right patient) | |
Workflow functions | Barcode driven processes such as case triage |
Provide status alerts to recipients about the location of an asset | |
Create flags for retrieval of interesting cases or cases for a specific function (e.g., such as tumor boards). |
An asset tracking system significantly contributes to reduction in labeling errors and thereby contributes to patient safety [3, 27]. Implementing a tracking solution to support work process standardization in the AP laboratory has been shown to resolve such issues. For example, at Henry Ford Hospital in Detroit, Michigan, pathologists reported a 62 % decrease of their overall misidentification case rate, 92 % decrease in slide misidentification defects, and 125 % increased technical throughput at their microtomy workstations after barcodes were introduced [15].
Even though the benefits of an asset tracking system are manyfold, there are challenges and barriers in the implementation of a tracking system. One of the major barriers is the immense cost, which in turn depends on the overall scope of the project [30]. Several software and hardware vendors offer customized solutions that will work with the LIS of your choice, or work as an independent tracking system outside of the LIS. Costs associated with the implementation of an asset tracking solution are not limited to hardware and software, but also require human resources, particularly from IT and laboratory teams, as well as consultants from the vendor (Table 5.7). In addition, there may be some indirect costs associated with configuring the implementation to change or accommodate the workflow needed for tracking and optimizing [3, 18].
Table 5.7




Costs that may be incurred in implementing an asset tracking solution in the laboratory
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


