Aortic Endografting
Gregorio A. Sicard
John A. Curci
Aortic Endografting
Endovascular repair has, over the past 20 years, become the predominant method for the elective treatment of abdominal aortic aneurysms (AAAs) in the United States. It decreases tissue trauma compared to open repair by delivering graft material mounted on stents (an endograft) to the infrarenal aorta and iliac vessels from remote incisions in the groin. Advances in device design and deployment have greatly increased the safety and durability of the procedure. This technique permits the sutureless aortic aneurysm exclusion by an endograft introduced through the femoral artery.
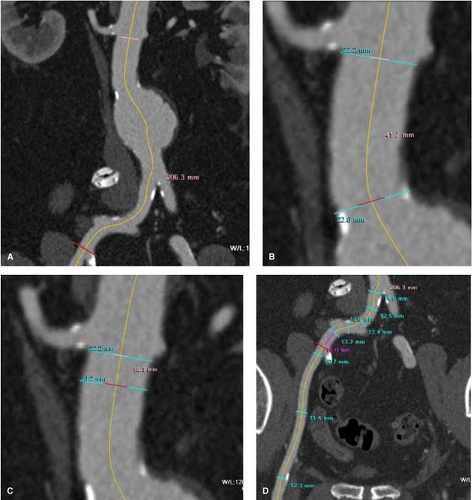
Endovascular aneurysm repair (EVAR) was initially reported by Dr Parodi and collaborators in 1990 as an alternative for patients deemed unfit for open aneurysm repair as a result of significant comorbidities. Exponential improvements in device design during the first 10 years, along with patient preference for a less-invasive approach, have significantly expanded its use. The introduction of EVAR as a good alternative to open repair has not changed the indications for the treatment of AAA. Different than open repair, the use of currently approved devices in the United States is limited to patients whose vascular anatomy meets the following requirements: (a) a proximal aortic sealing or “landing zone” diameter below the lowest renal artery that ranges between 18 and 32 mm and 15 mm or more in length (often referred to as the “neck” of the aneurysm) and (b) suitable iliac artery diameter to allow insertion of the device. In order to prepare for these cases preoperatively, these anatomic requirements demand precise preoperative imaging that permits accurate measurements of the diameter and length of the aorta from the level of the renal arteries to the common femoral arteries. This is best accomplished by thin-slice (1 to 3 mm) contrast abdominal and pelvic computed tomographic (CT) scan. Because of the tortuosity, which develops with the lengthening of the aorta as it dilates, precise diameter measurement can best be accomplished when the thin-slice CT images are reconstructed in a three-dimensional field, which allows for centerline measurement at the appropriate anatomic sites (Fig. 1A–D). Contrast CT scan can identify the presence or absence of thrombus, calcification, or significant atheromatous disease in the aortic neck (Fig. 1B, C) and/or iliac arteries (Fig. 1D) that may interfere with an adequate endograft seal. Three-dimensional reconstruction of the thin-slice CT scan has practically eliminated the need for preoperative angiography. In patients with iodinated contrast allergy, magnetic resonance angiography with gadolinium can be useful in providing all the anatomic information required.
There are currently five commercially available endografts in the United States: AneuRx and Talent (Medtronic Vascular, Santa Rosa, CA), Excluder (W.L. Gore, Flagstaff, AZ), Zenith (Cook, Inc., Bloomington, IN), and Powerlink (Endologix, Irvine, CA) (Table 1). All the current commercially available devices are based on self-expanding stents to produce radial force and add columnar strength. Each device has different construction in terms of the stent material (nickel–titanium alloy, cobalt–chromium alloy, or stainless steel), the type and thickness of the graft fabric used (Dacron, expanded polytetrafluoroethylene), as well as, different attachment mechanisms at the proximal and/or distal landing zones. Even
though all the devices rely on the radial force of the stent for attachment (AneuRx and Powerlink), some devices have added barbs or hooks (Excluder, Zenith) or a suprarenal uncovered extension to add to the proximal fixation (Zenith and Talent). To accomplish maximal expansion and appropriate sealing at the landing zones, the diameter of the endograft must be 10% to 20% larger at full expansion than the diameter of the proximal (infrarenal aortic) neck and distal iliac arteries. The surgeon must become familiar with the construction of the device, sheath size, delivery system characteristics, configuration, and the various diameters and lengths of the devices. In addition, the surgeon must follow the recommendations by each manufacturer in the instructions for use regarding maximal aortic neck diameter and length, as well as neck angulation (Table 1).
though all the devices rely on the radial force of the stent for attachment (AneuRx and Powerlink), some devices have added barbs or hooks (Excluder, Zenith) or a suprarenal uncovered extension to add to the proximal fixation (Zenith and Talent). To accomplish maximal expansion and appropriate sealing at the landing zones, the diameter of the endograft must be 10% to 20% larger at full expansion than the diameter of the proximal (infrarenal aortic) neck and distal iliac arteries. The surgeon must become familiar with the construction of the device, sheath size, delivery system characteristics, configuration, and the various diameters and lengths of the devices. In addition, the surgeon must follow the recommendations by each manufacturer in the instructions for use regarding maximal aortic neck diameter and length, as well as neck angulation (Table 1).
Most commercially available devices have a bifurcated design of the main body of the device, some require extension of the device into one or both iliac arteries and others require proximal extension of the graft to the level of the renal arteries. (The technique of endograft insertion is described later.) Tapered, tubular grafts, known as aortouniiliac configurations, are also available. When an aortouniiliac device is used, adjuvant means to deliver blood to the contralateral limb (typically a femoral–femoral bypass) as well as exclude retrograde flow into the aneurysm via the contralateral iliac with some type of occluder device.
Intraoperative Imaging
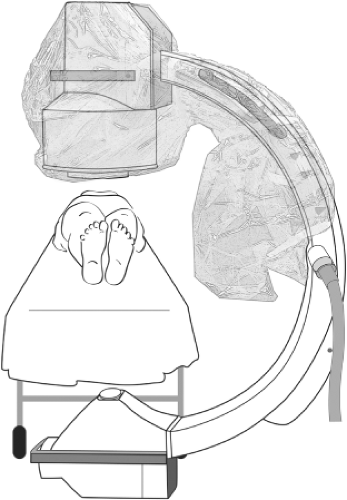
High-quality intraoperative imaging during an endovascular procedure is necessary for accurate placement of the endograft. Contrast-enhanced fluoroscopy is the primary imaging modality. A high-resolution, mobile C-arm can be used for the endovascular treatment of AAA in an operating room environment (Fig. 2). A fixed radiographic system can give superior imaging, and when combined with adequate operative lighting and anesthesia equipment can be a preferred venue for EVAR. Adjuvant imaging with intravascular ultrasound has also proved valuable in some individuals with renal insufficiency to limit contrast use.
Table 1 Commercially Available Endografts in the United States | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||
Patient selection based on anatomic criteria is the single most important indicator of success in EVAR. Careful attention needs to be paid to the details of the neck diameter and length, the distance from the renal arteries to the aortic bifurcation and the hypogastric arteries bilaterally, as well as the diameters of the common iliac and external iliac arteries. Each device has specific anatomic limitations, which affect delivery and sealing of the device within the aorta and iliac systems. Detailed familiarity with the characteristics of specific devices and their components is necessary to select the device best suited for an individual. The iliac through which the main body of the device will be passed as well as the specific components to be used during the procedure should also be planned in advance of the procedure.
Although a thorough preoperative cardiovascular assessment and medical optimization is important for any surgical procedure, EVAR can and is frequently used in patients deemed to be unfit for open repair. EVAR can be performed under local or spinal
anesthesia in a cardiopulmonary high-risk patient with excellent results.
anesthesia in a cardiopulmonary high-risk patient with excellent results.
Patients with a known or suspected contrast allergy should receive preoperative steroids (methyl prednisolone, 50 mg by mouth every 6 hours/four to six doses/24 to 36 hours/preprocedure) and diphenhydramine hydrochloride (50 mg by mouth, 1 to 2 hours) preoperatively. An intraoperative dose (Solu-Medrol, 100 mg) should be given.
 Fig. 3. Exposure of femoral arteries using a suprainguinal incision parallel to groin crease.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|