Imaging modality
Setting (labs vs intensive care units, interventional or operating room)
Feasibility (selective criteria; renal function)
Accuracy in wall evaluation and non mobile atheroma
Accuracy in wall evaluation and mobile atheroma
Panoramic imaging and data of the aortic vessels
Cost
Invasiveness
Contrast agents
TTE
All
++++
++
+
+
+
−
−
TEE
All
++++
++++
++++
++
++
+
−
Cardiac CT
RX laboratory
+++
++++
+
++++
+++
−
+
Cardiac MR
RX laboratory
+++
+++
++
++++
++++
−
+
Angiography
Interventional
+
+
+
+++
++++
++++
+
Epiaortic Echo
Operating room
+
++++
++++
+
++++
++
−
Transthoracic Echocardiography (TTE)
TTE allows assessment of the aortic root and the proximal ascending aorta but cannot adequately image aortic arch plaques [67, 68]. However, echocardiographic evaluation of the aorta is a routine part of the standard echocardiographic examination. Although TTE is not the technique of choice for overall assessment of the aorta, it is useful for the diagnosis and follow-up of some segments of the aorta. TTE is one of the techniques most used to measure proximal aortic segments in clinical practice [69, 70]. The long-axis view affords the best opportunity for measuring aortic root diameters by taking advantage of the superior axial image resolution. Of paramount importance for evaluation of the thoracic aorta is the suprasternal view (Figs. 1.1 and 1.2). This view primarily depicts the aortic arch and the three major supra-aortic vessels (innominate, left carotid, and left subclavian arteries), with variable lengths of the descending and, to a lesser degree, ascending aorta. Although this view may be obstructed, particularly in patients with emphysema or short, wide necks, it should be systematically sought if aortic disease is evaluated. From this window, aortic coarctation can be visualised and functionally evaluated by continuous-wave Doppler; a persistent ductus arteriosus may also be identifiable by colour Doppler. Dilatation and aneurysm, plaque, calcification, thrombus, or dissection membranes are detectable if image quality is sufficient. A systematic comparison of harmonic TTE and TEE made to detect aortic plaques and thrombi revealed high sensitivity for the detection of aortic arch atheromas protruding ≥4 mm into the lumen [2]. However, the entire thoracic descending aorta is not well visualised by TTE.
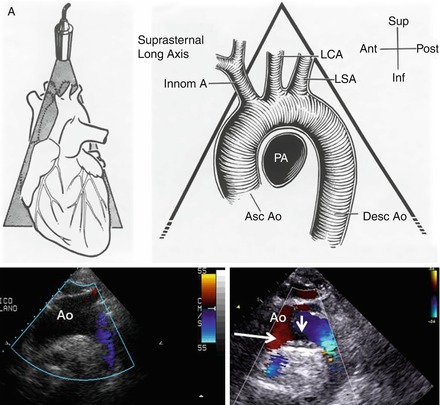
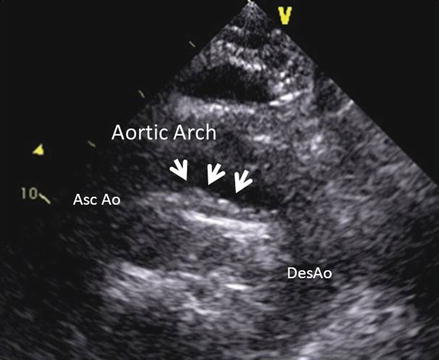

Figure 1.1
Schematic representation of the aortic arch as seen by transthoracic echo from the suprasternal view. The true echo images (bottom) show on the left a normal appearance of the arch and an example of on atheroma at the level of the ventral part of the arch (arrow)

Figure 1.2
Example of an atheroma of the aortic arch (arrows) visualized from the suprasternal view by transthoracic echo
Transoesophageal Echocardiography (TEE)
TEE allows us not only to assess plaque mobility, ulceration, and composition [71], but also to obtain detailed information on the anatomic relationship between the plaque location and the origin of the large arterial branches [12, 25, 32, 72–76].
Beside the possibility of structural damage, other limitations associated with this diagnostic technique include the need for conscious sedation and patient cooperation for swallowing the probe, both quite difficult in patients with stroke [71]. In addition, due to the shadow associated with the tracheal air column near the origin of the innominate artery, which masks a portion of the ascending aorta when monoplanar and biplanar probes are used, an estimated 2 % of plaques are missed [77]. The use of multiplanar probes may reduce this problem [78] (Fig. 1.3).
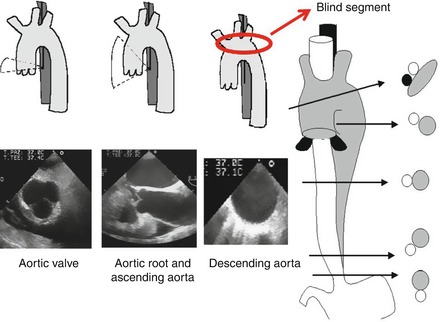

Figure 1.3
Schematic representation of the thoracic aorta and relative position of the transoesophageal echo probe inside the oesophagus. The entire aorta may be visualized by gentle rotation of the probe anteriorly or posteriorly. Bottom: corresponding TEE examples of the short axis of the aortic valve (bottom left), long axis of the proximal and ascending aorta (bottom middle) and short axis of the descending aorta (bottom right). The red circle and arrow show the blind segment of the aortic arch due to the interposition of the left bronchus
The presence of detectable atherosclerotic plaques in the aorta indicates the presence of atherosclerotic disease and is a possible source of embolism [12]. Aortic atheromas are characterised by irregular intimal thickening of at least 2 mm, with increased echogenicity. They often have superimposed mobile components, mainly thrombi. The morphology of atheromatous plaques is dynamic, with frequent formation and resolution of mobile components [45]. TEE is the imaging modality of choice for diagnosing aortic atheromas. It provides higher-resolution images than TTE and has good inter-observer reproducibility [79]. The prevalence of aortic atheromas on TEE varies depending on the population studied. In a community-based TEE study [80], aortic atheromas were present in 51 % of the population over 45 years, being complex in 7.6 %. Atheroma prevalence increased with age, smoking, and pulse pressure. TEE characterises the plaque by assessing plaque thickness, ulceration, calcification and superimposed mobile thrombi, thereby determining the embolic potential of each plaque. The advantages of TEE over other non-invasive modalities include its ability to assess the mobility of plaque in real time. The French Aortic Plaque in Stroke group showed that increasing plaque thickness of ≥4 mm is associated with a significantly increased embolic risk [39]. They used TEE to characterise aortic arch plaque thickness in 331 patients older than 60 years with stroke. Increasing plaque thickness was associated with an increased risk of embolic events. The odds ratio for aortic arch plaque <1 mm and stroke was 1.0. The odds ratio was 3.9 for plaque between 1 and 3.9 mm thick and 13.8 for plaques >4 mm thick. The presence of mobile lesions (thrombi) superimposed on aortic atheromas has been recognized to imply a high embolic risk. Other characteristics of the lesions seen on TEE, such as ulceration ≥2 mm in aortic plaques and no calcified plaques, were also associated with a higher risk of stroke [81]. Thus, atherosclerotic plaques are defined as complex in the presence of protruding atheromas of 4 mm in thickness, mobile debris, or the presence of plaque ulceration, and defined as simple if the plaques lack these morphological features (Fig. 1.4).
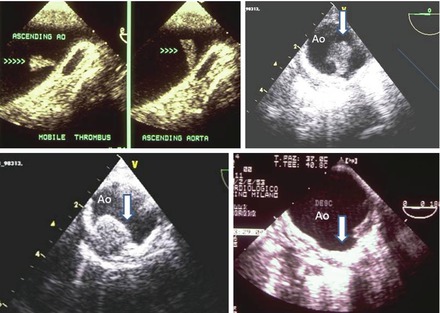

Figure 1.4
Examples of TEE images of different cases of thoracic aorta pathologies that may be correlated to embolic events. Top left: TEE of the distal ascending aorta showing a mobile thrombus attached to aortic wall (arrows indicate the mobility of the thrombus in two different frames). Top right: TEE of the descending aorta. In this short axis a very large protruding thrombus attached to a large aortic plaque is clearly detected. Bottom left: TEE of the descending thoracic aorta. Large protruding atheroma of the aortic wall. Bottom right: TEE of the descending thoracic aorta: large ulcerated plaque with a crater like appearance (arrow)
A grading system for severity of atherosclerosis in the thoracic aorta has been proposed by Montgomery. Grade I = normal or minimal intimal thickening; Grade II: extensive intimal thickening; Grade III = atheroma <5 mm; Grade IV = atheroma ≥5 mm; Grade V = mobile lesion (Fig. 1.5).
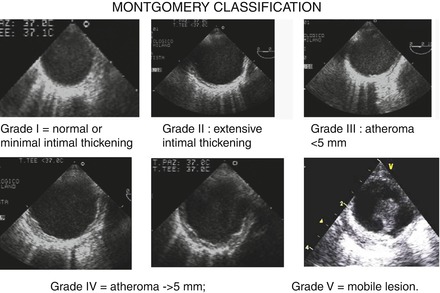

Figure 1.5
Grading system for severity of atherosclerosis in the thoracic aorta proposed by Montgomery. Grade I = normal or minimal intimal thickening; Grade II: extensive intimal thickening; Grade III = atheroma <5 mm; Grade IV = atheroma ≥5 mm; Grade V = mobile lesion. All examples refer to short axis of the descending aorta
Two recent community-based studies found no association between aortic atheromas and future stroke [59, 80]. An alternative explanation is that atheromatous plaque is merely a marker for diffuse atherosclerosis that predisposes patients to systemic embolism by other cardiovascular mechanisms. The embolic potential of atherosclerotic aortic lesions during invasive procedures or during open-heart surgery is well established [82, 83]. Some studies have shown the risk of stroke or peripheral embolism after cardiac catheterization or intra-aortic balloon pump placement in patients with severe aortic atherosclerosis diagnosed by TEE [82]. A strong association between aortic stenosis and aortic atherosclerosis has recently been established [84]. The presence of plaques in the aorta of patients with aortic stenosis has important implications since these patients often undergo invasive diagnostic and therapeutic procedures that can dislodge particularly thick plaques and the attached thrombotic material. Large mobile aortic thrombi are possible causes of systemic emboli and appear to be a complication of atherosclerosis. TEE is the best technique for diagnosing and monitoring the evolution of these large thrombi [85]. Figure 1.6 shows a three-dimensional image of large complex plaques in the thoracic aorta of a patient undergoing TAVI for severe aortic stenosis.
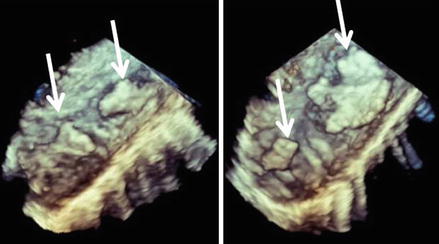

Figure 1.6
3D transoesophageal imaging of atheromas of the descending aorta. Arrows show several athoromas in two segments of the thoracic descending aorta visualized in a longitudinal view. 3D imaging allows a more detailed view of the complexity of plaques which are irregular in shape and extension
Epiaortic Ultrasonography
Magnetic Resonance (MR) Imaging
MR may be used for detection and measurement of aortic arch plaque [77, 88] and to monitor aortic plaque progression and regression [86]. Contrast MR is also used to identify morphological features such as calcification, thrombus, fibrocellular tissue and lipids which may be useful to detect plaque stability.
Contrast MR angiography may underestimate the plaque thickness and its use may be limited in obese or claustrophobic patients as well as in subjects who have metallic implants.
Computed Tomography (CT)
Multidetector CT has been demonstrated to be an accurate and powerful tool for detecting atheroma in extra- and intracranial vessels. CT is the test of choice for detecting vascular calcification and can reliably detect and measure protruding aortic plaques [77, 89]. CT identifies more plaques throughout the aortic arch and around the origins of the major cerebral arteries in particular compared to TEE (Fig. 1.7). These may represent potential embolic sources of acute ischaemic stroke. Better plaque detection may have an impact on the best available secondary prevention regimen in individual patients if proximal embolic sources are suspected. MDCT allows evaluation of the whole arterial vasculature. In addition, MDCT has the ability to visualize the vessel wall and to give a quantitative measurement of calcified and noncalcified plaque.
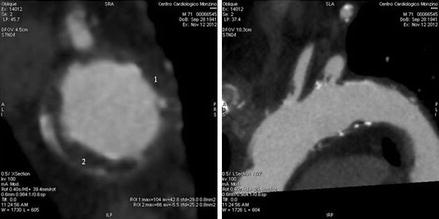

Figure 1.7
Atheromas of the aortic arch visualised by cardiac computed tomography; the white small spots visualised in the short (left) or long (right) images indicated calcified deposition inside the aortic wall, while the small diffused indentation of the walls (black spots) are due to parietal atheromas
However, CT may underestimate the amount of non-calcified plaque and mobile thrombus that presumably is at high risk for embolisation [77]. In conjunction with positron emission tomography, it can be used to localise fluorodeoxyglucose uptake by the plaque, identifying active plaques and plaques prone to rupture (unstable) [90], but its clinical utility has yet to be established.
Concerning specifically pre-operative (cardiac surgery) evaluation, preoperative CT scans in patients at high risk may help identify aortic areas at risk before entering the operating room, lead to more thorough screening in the operating room, and result in a more aggressive strategy to avoid calcified areas.
Microembolic Signals by Transcranial Doppler (TCD) Ultrasonography
The occurrence of microembolic signals, as detected by transcranial Doppler (TCD) ultrasonography, can be a marker of severe aortic atherosclerosis, and monitoring these signals should enable the application of appropriate surgical methods to coronary artery bypass patients who are at higher risk of stroke [91]. Asymptomatic cerebral embolic signals (ES) may be associated with severe (≥4 mm) aortic arch atherosclerosis but not with aortic arch atherosclerosis <4 mm or no aortic arch atherosclerosis [92].
Contrast Aortography
The need for contrast injection and radiation as well as the risk of iatrogenic complications due to the invasive nature of the procedure make this technique rarely used to assess aortic plaques [93].
Treatment of Aortic Atheroma
There are no published and conclusive randomised trials to guide the therapy of aortic embolism. So, only strategies for general atherosclerosis management are usually recommended.
Beside nonspecific risk modification (e.g. smoking cessation), strategies include medical, surgical, and interventional therapy.
Medical Therapy
Treatment of Aortic Atheroma Without Embolic Events
As both types of aortic embolisms (thromboembolism and atheroembolism) occur more frequently in the context of atherosclerosis, strategies devoted to the control of atherosclerosis risk factors, including smoking cessation and pharmacological control of conventional risk factors such as blood pressure, lipids and diabetes, may indirectly prevent the embolism from aortic plaques. Even if ad hoc clinical trials are needed to determine the effects of anti–atherosclerotic and antiplatelet treatments in patients with severe aortic atheroma and risk of atheroembolism, on the basis of general guidelines for primary and secondary prevention of atherosclerosis–related events, proposed pharmacological therapies for aortic embolism include statins and antiplatelet agents.
Statins
A body of indirect evidence, obtained in a variety of patient cohorts, suggests that statins may reduce the risk of stroke [4, 94]. For example, in an observational study of 519 patients with severe aortic plaques documented by TEE, statin use resulted in a relative risk reduction for ischaemic stroke of 59 % [64].
Plaque regression in the thoracic aorta and retardation of plaque progression in the abdominal aorta by 1-year atorvastatin (20 mg versus 5 mg) have also been reported in an MRI prospective, randomised, open–label trial carried out in 36 hypercholesterolaemic patients [95]. After 2 years of treatment, regression of thoracic plaques was found in the 20 mg group (−15 % vessel wall area reduction), but not in the 5 mg group (+7 %). Regarding abdominal plaques, progression was found in the 5 mg group (+10 %), but not in the 20 mg group (+2 %). The degree of thoracic plaque regression correlated with LDL–cholesterol reduction (r = 0.61), whereas thoracic plaque change from 1 to 2 years correlated with on–treatment LDL–cholesterol levels (r = 0.64). In the abdominal aorta, only retardation of plaque progression was found after 2 years of 20 mg treatment [95].
The incidence of stroke may be reduced by statins not only through their conventional lipid-lowering effect, but also with other mechanisms; the so called “pleiotropic effects”, which include plaque regression/stabilisation, reduction in the inflammatory response, and inhibition of the coagulation cascade at different levels. For example, a regression of thoracic aortic plaques after lipid–lowering therapy with simvastatin was demonstrated using MRI [96, 97]. Other two MRI randomized studies evaluated the effects of aggressive vs conventional lipid-lowering therapy in patients with aortic and/or carotid plaques and reported significant plaque regression which was significantly associated with the reduction in LDL cholesterol [98, 99]. In one of the two studies, a relationship with the statin dosage was also observed [99].
On these bases, despite the lack of ad hoc randomized trials, there are guidelines clearly stating that: “a treatment with a statin is a reasonable option for patients with aortic arch atheroma to reduce the risk of stroke” [8] (Table 1.2). In addition, it has to be kept in mind that statins are recommended for many manifestations of atherosclerotic diseases; therefore, a large number of patients with identified aortic plaque already have indications for statins for secondary prevention for cerebrovascular diseases [100].
Table 1.2
Treatment recommendations for uncomplicated aortic atheroma, complicated atheroma, suspected aortic embolic event, large thrombi
Therapy | Recommendations | Classa | Levelb |
|---|---|---|---|
Uncomplicated atheroma | |||
Statin is a reasonable option for patients with aortic arch atheroma to reduce the risk of stroke | Recommended [8] | IIa | C |
Oral anticoagulation therapy with warfarin (INR 2.0–3.0) or antiplatelet therapy | Reasonable in stroke patients with aortic arch atheroma 4.0 mm or greater to prevent recurrent stroke [8] | IIb | C |
Patients with thoracic aortic atheroma, not requiring surgery, as well as for patients who are not considered to be surgical or stent graft candidates | |||
Stringent control of hypertension | I | B/C | |
Lipid profile optimization | Recommended [8] | IIA | A |
Smoking cessation, and other atherosclerosis risk-reduction measures | Recommended [8] | I | B |
Patients with thoracic aortic atheroma undergoing cardiac surgical procedures requiring cardiopulmonary bypass (coronary bypass surgery and valve surgery) | |||
Endarterectomy of aortic arch plaque for the purposes of secondary stroke prevention | not recommended [101] | ||
Complicated aortic atheroma and/or suspected aortic embolic event and/or Large aortic thrombi | |||
Patients with an ischemic stroke or TIA and evidence of aortic arch atheroma | |||
Antiplatelet therapy | Recommended [102] | I | A |
Statin therapy | I | ||
Anticoagulation with warfarin | Effectiveness unknown [102] | IIB | C |
Endarterectomy of aortic arch plaque for the purposes of secondary stroke prevention | not recommended [102] | III | C |
Patients with native aortic disease who do not have AF or another indication for anticoagulation | |||
Antiplatelet therapy | Recommended [102] | I | C |
Anticoagulation Versus Antiplatelet Therapy
Coexistent severe aortic atherosclerosis could affect prothrombotic profiles of patients with atrial fibrillation (AF). Being at risk for thromboembolism, such patients seem to have an indication for an intensive antithrombotic therapy [104]. Patients with nonrheumatic AF who have spontaneous echocardiographic contrast in the descending thoracic aorta appear to have enhanced coagulation activity but not platelet activity [105]. Cholesterol emboli on skin, muscle, and renal biopsy samples often occur in patients with aortic arch atheroma [42], but there is also evidence, even if coming from small scale studies, which shows that the prevalence of clinical atheroemboli syndrome is lower when patients are treated with warfarin, thus suggesting a potential benefit of this therapy in patients with aortic arch atheroma.
A second observational TEE study [106] was carried out in 129 patients with aortic arch atheroma to investigate the source of cerebral or peripheral embolisation. In this non-randomised study, the antithrombotic therapy (oral anticoagulation, aspirin, or ticlopidine) was left to the discretion of the practitioner in charge of the patient. At the end of follow-up (22 ± 10 months), a significant reduction in the number of embolic events among patients who received oral anticoagulants (0 events in 27 patients versus 5 events in 23 patients treated with antiplatelet agents) was noted even if just in patients with plaques ≥ to 4 mm.
The SPAF (Stroke Prevention in Atrial Fibrillation) trial [61] was a randomised study carried out to define TEE predictors of stroke in patients with atrial fibrillation and to examine response to antithrombotic therapy. A total of 382 patients with atrial fibrillation at high risk for thromboembolism have been included into the study. Among patients with “high–risk” non–valvular atrial fibrillation, the 1-year risk of stroke in 134 patients with complex aortic plaque was found to be reduced from 15.8 % (11 events) in those treated with fixed low–dose warfarin plus aspirin (INR 1.2–1.5) to 4 % (3 events) in those treated with adjusted–dose warfarin (INR 2.0–3.0), which means a 75 % relative risk reduction (P = 0.02) for patients with atheromas who received “therapeutic range” anticoagulation [61].
All these reports suggest that warfarin in patients with aortic arch atheroma is not harmful and may reduce the rate of stroke. Therefore, intensive anticoagulation treatment seems to be a reasonable option for patients with aortic atheroma and the 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines [8] suggest that oral anticoagulation therapy with warfarin (INR 2.0–3.0) or antiplatelet therapy may be considered in stroke patients with aortic arch atheroma 4.0 mm or greater to prevent recurrent stroke.
Despite these indications, the use of anticoagulation in patients with aortic plaques, even in those with thrombogenic mobile components, is still controversial. Indeed, retrospective information show no significant benefit of warfarin or antiplatelet drugs on the incidence of stroke and other embolic events in patients with severe thoracic aortic plaques on TEE [64]. In addition, in other small scale studies, either harmful or beneficial effects of anticoagulation were reported and anticoagulation has been associated with both worsening [21, 107], and improvement of an aortic thrombus in a patient with the atheroemboli syndrome [108]. Moreover, the use of warfarin in patients with aortic atheroma may be harmful also because of the theoretical risk of plaque haemorrhage, which may result in atheroemboli syndrome (i.e. blue toes, renal failure and intestinal infarction) [109].
It has to be underlined that all the studies published so far were not randomised trials and that they are not specifically designed for treatment of patients with aorta atheromas, and that the sample size is always relatively small. So, the ideal therapeutic approach to these patients still awaits prospective randomized double blind evaluation.
As for statins, it has to be underlined, however, that many patients with aortic embolism already have a strong indication for the use of antiplatelet agents for secondary prevention of cardio- and cerebrovascular events.
Thrombolytic Agents
In acute cerebral thromboembolism, thrombolytic agents may be used to restore blood flow in the affected vessel. There is evidence showing that mobile aortic atheroma may disappear with the use of these drugs [110].
Treatment of Aortic Atheroma After Stroke or Suspected Embolic Event
Statins
Despite the absence of randomized clinical trials specifically designed to evaluate the effectiveness of statin therapy for reducing the risk of recurrent stroke among patients with complex aortic plaque, observational studies in patients with a recent embolic event, including stroke or TIA, suggest that this class of drugs may be effective in preventing recurrent events [64]. On these basis, recent guidelines [102, 103] recommend statins to reduce the risk of stroke and cardiovascular events among patients with ischemic stroke or TIA who have evidence of aortic atherosclerosis (Table 1.2).
Anticoagulation Versus Antiplatelet Therapy
Data on the use of anticoagulant or antiplatelet therapy for secondary prevention of atheroembolism are quite inconsistent. As for statins, also in this case randomized clinical trials are lacking, and the observational studies available are small and results mixed [25, 81, 106, 111].
The ARCH (Aortic Arch Related Cerebral Hazard) trial is the unique open–label trial testing the usefulness of full–dose anticoagulation with warfarin (target INR 2.0–3.0) against a combination of low–dose aspirin (75 mg/day) plus clopidogrel (75 mg/day) for prevention of recurrent vascular events in patients with aortic atheroma (4 mm or greater) and non–disabling stroke [112]. Unfortunately, although completed, the results of this study have not yet been published (Table 1.2).
Endarterectomy or Cover Stents
In a study designed to assess the risks or benefits of aortic arch endarterectomy for reducing the risk of recurrent aortic atheroembolism and intraoperative stroke during cardiac surgery provided unpromising results [14]. Arch endarterectomy was performed in 43 of 268 patients who had arch atheromas ≥5 mm or with mobile components on intraoperative TEE [14]. The overall mortality and the incidence of intraoperative stroke were rather high (14.9 % and 15.3 %, respectively) and, on multivariate analysis, aortic arch endarterectomy was even an independent predictor of intraoperative stroke (OR, 3.6; P = 0.001). A possible alternative to endarterectomy to prevent embolization might be the use of cover stents, which may have the potential advantage of shielding severely diseased aortic segments. Unfortunately, interventional endovascular manipulations or diagnosis may induce periprocedural embolization. As a consequence also in this case there is no sufficient evidence to recommend prophylactic endarterectomy or aortic arch stenting for purposes of stroke prevention, and therefore surgical guidelines for the management of thoracic aortic disease do not recommend prophylactic endarterectomy or aortic arch stenting for purposes of stroke prevention [101] (Table 1.2).
Treatment of Large Aortic Thrombi
Large Mobile Aortic Thrombi
Mobile thoracic or abdominal thrombi in a nonaneurysmal, minimally atherosclerotic or normal aorta is a rare clinical entity and an uncommon cause of embolism to visceral organs or lower limbs [113–117]. These may be the consequence of underlying pathology such as hypercoagulable disorders [113, 118, 119], concurrent malignancy [113], periprocedural outcomes [120], anticancer treatments e.g. cisplatin-based chemotherapy [121], Crohn’s Disease [122], protein C deficiency [123], essential thrombocytosis [124], and traumatic aortic injury [125]. The discovery of an aortic thrombus may be even incidental [126].
Because of the rarity of this condition there is a paucity of case reports. Both anticoagulation therapy and aortic surgery or both [127, 128], are commonly used as primary treatment, but there are no consensuses or clinical guidelines to outline the best management strategy for this unusual problem. No long-term follow up of this rare pathology is available.
According to a number of case report studies endovascular stent graft placement is feasible and can be performed as an effective and minimally invasive treatment option for mobile thoracic aortic thrombi [129–137]. Surgical removal of the aortic thrombus may be another options [114, 117, 123, 138–144].
Treatment strategy for thrombus originating from an almost normal thoracic aorta remains controversial [145]. According to some authors, the indication for surgical intervention results from contraindication to anticoagulation, mobile thrombus or recurrent embolism. Whenever possible, endovascular therapy should be preferred [113].
Anticoagulants
In studies carried out in patients with aortic mobile lesions documented by TEE [25, 146], the authors described an incidence of vascular events much lower in patients treated with warfarin than in those who were not treated (5 % versus 45 %). In addition, mobile aortic atheromas have been noted to disappear during anticoagulant therapy, in some [34, 108, 147], but not all studies [148]. In the SPAF trial [61] the trend toward fewer embolic events while on anticoagulants in patients with mobile lesions, did not reach statistical significance, whereas the mortality was significantly reduced [61].
A systematic review including a meta-analysis including 98 articles compares the outcomes of anticoagulation therapy and aortic surgery strategies for the treatment of aortic mural thrombus. Two hundred patients were considered: 112 received anticoagulation and 88 underwent aortic surgery as primary treatment. The results of the meta-analysis seem to favor the surgical management of aortic mural thrombus in the normal or minimally diseased aorta. Indeed, although mortality rates were similar (6.2 % and 5.7 % for the anticoagulation group and the surgery group, respectively; P = 0.879), anticoagulation as primary therapy was associated with a higher likelihood of recurrence of peripheral arterial embolization (25.7 % Vs. 9.1 %; P = 0.003), a trend toward a higher incidence of complications (27 % Vs. 17 %; P = 0.07), a higher likelihood of major limb amputation (9 % Vs. 2 %; P = 0.004). In addition, aortic thrombus persisted or recurred in 26.4 % of the anticoagulation group and in 5.7 % of the surgery group (P < 0.001). Multivariable logistic analysis established thrombus location in the ascending aorta or arch, mild atherosclerosis of the aortic wall and stroke presentation as important predictors of recurrence. On these basis the authors concluded that aortic surgery should be considered as primary treatment, particularly for those patients at high risk for recurrence considered to be good operative candidates [149].
Thrombolysis
Some case report studies suggest thrombolysis as another possible strategy to treat aortic thrombi [150, 151].
In conclusion in the literature there is no consensus how to treat a symptomatic floating aortic thrombus. This report shows that therapeutic strategies are influenced by the localisation of the thrombus, the co-morbidities of the patient and the physician’ s preferences. Endovascular treatment in combination with high dose statins has become the preferred treatment although long-term data are lacking [115].
Large Fix Aortic Thrombi
To date, there are no evidence-based data to support specific drug therapy for a patient with atheroembolism and large fix or mobile aortic thrombi. It makes sense to use statins in any patient with atherosclerosis, as these drugs have been shown to reduce the risk of myocardial infarction and stroke, and have a theoretical benefit on plaque stabilization. Surgical treatment should be considered for patients with abdominal aortic or popliteal artery aneurysms and downstream atheroembolism. There are case reports of atheroemboli in patients worsening after given warfarin or heparin. For this reason, some institutions are reluctant to prescribe these drugs for patients with atheroemboli or thromboemboli from aortic plaque. However, the incidence of this complication is quite low. Similarly, the current state of knowledge does not allow for selecting specific pharmacologic intervention in patients with thromboemboli from fix aortic plaque. Statin therapy does make sense, as these drugs theoretically stabilize plaques and prevent plaque hemorrhage, thrombosis, and subsequent embolization. Unstable aortic plaques may develop superimposed thrombi (red thrombi on pathologic examination), easily seen as mobile elements on TEE. Therefore, it is possible that anticoagulation with warfarin might prevent embolic events in these patients. For this reason, we are often in the position of recommending warfarin therapy for patients with emboli and severe atheromas seen on TEE, especially when superimposed mobile thrombi are seen. There are small series in the literature that indicate the potential benefit of warfarin.
Large fix aortic thrombi are a very common finding particularly in old patients with conventional and non conventional atherosclerotic risk factor. As previously underlined plaque thickness of ≥4 mm is associated with a significantly increased embolic risk. In an MRI study in aortic and carotid lesions on the effects of aggressive versus conventional lipid lowering therapy by simvastatin, treatment was associated with a significant regression of atherosclerotic lesions. Plaque regression was more related to the degree of LDL-C reduction rather than to the dose of statin, because no difference between high- and moderate dose simvastatin was detected.
Surgical and Interventional Therapy
In acute aortic thromboembolism, interventional approaches may be used to treat end–organ ischaemia (e.g. percutaneous intervention to restore the flow of a cerebral artery occluded by a thromboembolus). Covered stents, endarterectomy and even arterial bypass surgery have been used in a small number of patients with aortic embolism with limited success. Some authors suggest that mural aortic thrombi can be successfully treated with a definitive surgical procedure in selected patients, with low mortality and morbidity [57]. Despite this, at the moment there is no convincing evidence to recommend either endarterectomy or aortic stenting for stroke prevention in patients with thoracic aortic plaques [8]. Indeed, although a handful of case reports on aortic arch endarterectomy in patients with thromboembolism originating from aortic arch atheroma have reported successful results, this procedure seems to be associated with a relatively high rate of perioperative stroke and mortality (34.9 % with endarterectomy versus 11.6 % without endarterectomy), especially when performed to limit stroke during cardiac surgical procedures requiring cardiopulmonary bypass (coronary bypass surgery and valve surgery) [14]. Even if covered stents may offer the potential advantage of shielding severely diseased aortic segments to prevent further embolisation, peri–procedural embolisation may occur during diagnosis or interventional endovascular manipulations. Endovascular stenting for treatment of aortic embolism has largely been limited to abdominal aortic and infrainguinal forms of the disease [134].
Randomised blinded controlled trials are therefore needed to test currently available treatment options, both medical and surgical, to prevent embolic vascular events.
Follow-up of Aortic Atherosclerosis or Large Thrombi
No data exist in this filed. Large fix aortic plaques are a very common finding particularly in old patients with conventional and non conventional atherosclerotic risk factor and are associated in several cases to stratified thrombi. As previously underlined plaque thickness of ≥4 mm is associated with a significantly increased embolic risk. Therefore an aggressive treatment with anticoagulant plus statins may be reasonably advocated even though data on this topic are lacking.
DRUG Interactions
As stated before, proposed pharmacological therapies for aortic atheroma include mainly statins, anticoagulation and antiplatelet agents. To use these drugs in the most effective and safest manner, it could be useful for clinicians to have an understanding of mechanisms of potential drug interactions, which drug interactions have already been described and which drug interactions may at least theoretically occur. Since it would be impossible to describe all potential interactions, only those potentially occurring among statins, anticoagulants and antiplatelet agents will be described.
Interactions Affecting Lipid-Lowering Drugs
Interactions Affecting Statins
Statin interactions may have either a pharmacokinetic or pharmacodynamic basis, or both (Table 1.3) and it is now widely accepted that knowledge of the pharmacokinetic and pharmacodynamic properties of statins should avoid the majority of drug interactions. Pharmacokinetic interactions may be associated with adverse events such as myopathy and rhabdomyolysis, and to either an enhanced or reduced lipid lowering effect. Pharmacokinetic interactions may take place at any stage of absorption, distribution, metabolism, or excretion. An important role in statin metabolism is played by the cytochrome P450 (CYP) enzyme system; however, a level of difficulty in predicting possible statin interactions lies in the fact that different statins are metabolised by different CYP enzymes and to different degrees and in some cases the metabolism produces active metabolites. Lovastatin, simvastatin and atorvastatin are metabolised by the CYP3A family. Fluvastatin is metabolised by CYP2C9 whereas pravastatin is not significantly metabolised by the CYP system [166]. If concurrent therapy with known inhibitors of statin metabolism is needed, patients should be monitored for signs and symptoms of myopathy or rhabdomyolysis possibly reducing the dosage or even discontinuing the statin therapy if needed [166].
Table 1.3
Effects of co–prescribed drugs interfering with the absorption, metabolism or pharmacodynamic of statins
Co–prescribed treatment | Statin | Possible effects |
|---|---|---|
Interference with absorption | ||
Bile–acid sequestrants | Pravastatin [154] Simvastatin [155] Fluvastatin [156] | Absorption reduction |
Dietary fibre | Lovastatin [157] | Absorption reduction |
Oral antacid containing magnesium and aluminium hydroxides | Rosuvastatin [158] | Plasma concentration reduced |
Reduction of gastric acidity with H2-receptor antagonist (Cimetidine, ranitidine) Proton pump inhibitor (omeprazole) | Fluvastatin [152] | |
Food | Pravastatin [161] Atorvastatin [163] | Systemic bioavailability reduced (poor clinical significance) |
Food | Lovastatin [164] | Absorption increased (poor clinical significance) |
Herbal medicines (St. John’s wort) | Simvastatin [165] | Plasma concentration reduced |
Herbal medicines (wheat bran) | Lovastatin [165] | Plasma concentration reduced |
P-glycoprotein inhibitors | Statins [166] | Influence on oral bioavailability |
Aspirin | Statins [167] | No pharmacokinetic interactions |
Dual antiplatelet therapy (aspirin and clopidogrel) | No pharmacokinetic interactions | |
Ticagrelor | Statin plasma concentration modestly augmented (atorvastatin) or larger augmented (simvastatin) | |
Interference with the metabolism | ||
CYP3A4 inhibitors (e.g. cyclosporin) | Statins [166] | Increased statin exposure possibly leading to myopathy and rhabdomyolysis |
Itraconazole | Simvastatin [172] Statins [173] | Increased statin exposure possibly leading to myopathy and rhabdomyolysis |
Azole antifungals, macrolides, azalides, HIV protease inhibitors | Clinically significant interactions may occur | |
Pharmacodynamic interactions (may lead to either synergism or antagonism) | ||
Gemfibrozil | Cerivastatin [166] | Rhabdomyolysis |
Aspirin | Statins [176] | Positive interaction: additive effect in reducing cardiovascular events |
Thienopyridines (ticlopidine and clopidogrel) | Recommendation in [179] | |
Clopidogrel | No relevant interactions | |
Clopidogrel | Diminished anti platelet activity of clopidogrel | |
Prasugrel | No relevant interactions | |
Of course, statins may also affect the metabolism and consequent concentrations of other coadministered therapies, such as digoxin, leading to alterations in effect or a requirement for clinical monitoring.
Warfarin Drug–Drug Interactions
Warfarin can interact, at least potentially, with a large number of drugs by both pharmacokinetic and pharmacodynamic mechanisms (Table 1.4). Warfarin is a racemic mixture of S and R isomers. The two isomers are metabolized by two different cytochrome P450 enzymes: CYP2C9 (which metabolizes the S isomer) and CYP3A4 (which metabolizes R isomer). As the S isomer is five times more potent than the R isomer, drug interactions involving the S isomer have usually a higher clinical importance. Knowledge on whether co–prescribed drugs are metabolized by these two CYP enzymes allows a reasonable INR change prediction and possible subsequent bleeding (Tables 1.5 and 1.6).
Table 1.4
Effects of co-prescribed drugs interfering with the metabolism or the clearance of warfarin
Mechanism | Examples | Possible effects |
|---|---|---|
CYP2C9 induction | INR reduction | |
CYP2C9 inhibition | strong INR increase | |
CYP3A4 induction | St John’s wort [202] | INR reduction |
CYP3A4 inhibition | Clarithromycin [199] | INR increase |
Interference with the clearance of warfarin | Underlying condition for which the antibiotic is prescribed (eg. Pneumonia) [204] | INR increase |
Interference with clearance of the R isomer of warfarin | Modest INR increase | |
Interference with the clearance of both warfarin isomers | Strong INR increase | |
Interference with the amount of vitamin K produced by the intestinal bacteria | INR increase | |
Reduction of vitamin K intake | Dietary changes associated to antibiotics treatments [204] | INR increase |
Table 1.5
Examples of substrates, inhibitors, and inducers of CYP2C9
Substrates | Inhibitors | Inducers |
|---|---|---|
NSAIDs (analgesic, antipyretic, anti–inflammatory): Celecoxib [212] Lornoxicam [213] Diclofenac [212] Ibuprofen [212] Naproxen [212] Piroxicam [212] Meloxicam [213] Suprofen [212] Flurbiprofen [212] Mefenamic acid [212] | Strong: | Strong: |
Antifungal azoles: Fluconazole [214] Miconazole [214] Voriconazole [214] | Rifampicin [214] (bactericidal) | |
Dexamethasone [214] (Glucocorticoid) | ||
Bosentan [214] (endothelin receptor antagonist) | ||
Valproic acid [214] (Anticonvulsant) | Phenobarbital [214] (Barbiturate) | |
Sulfamethoxazole [214] (Antibacterial) | Anticonvulsants, mood stabilizers: Carbamazepine [214] Phenytoin [214] | |
Imatinib [214] (tyrosine–kinase inhibitor) | ||
Gemfibrozil [214] (fibrate) | Unspecified potency: | |
Zafirlukast [214] (leukotriene antagonist) | St. John’s Wort [215] | |
Fluvastatin [212] (Statin) | Amiodarone [214] (Antiarrhythmic) | Secobarbital [216] (Barbiturate) |
Ketamine [217] (Sedative) | Fluvastatin [214] (Statin) | |
Terbinafine [214] (Antifungal) | Weak: | |
Angiotensin II receptor antagonists (in hypertension, diabetic nephropathy, CHF): | Clopidogrel [214] (antiplatelet agent) | |
Irbesartan [212] | Curcuma [214] | |
Losartan [212] | Irbesartan [214] (Angiotensin II receptor antagonist) | |
Sulfonylureas (antidiabetics): Glipizide [212] Glimepiride [212] Tolbutamide [212] Glyburide [212] (or glibenclamide) | Fluvoxamine [214] (Selective serotonin re–uptake inhibitors or SSRI) | |
Unspecified potency: | ||
Amentoflavone [218] (constituent of Ginkgo biloba and St. John’s Wort) | ||
Other antidiabetics: | Phenylbutazone [219] (NSAID) | |
Nateglinide [214] | Flavones or flavonols: [220] | |
Rosiglitazone [212] | Quercetin [220] | |
S–warfarin [212] (Anticoagulant) | Luteolin [220] | |
Phenytoin [212] (Antiepileptic) | Baicalein [220] | |
Cyclophosphamide [212] (Alkylating agent) | Wogonin [220] | |
Apigenin [220] | ||
Sildenafil [212] (in erectile dysfunction) | Sulfaphenazole [216] (Antibacterial) | |
Torasemide [212] (Loop diuretic) | H1–receptor antagonists (Antihistamines): | |
Amitriptyline [214] (Tricyclic antidepressant)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|