Figure 9-1. Overlap of major depressive disorder and anxiety disorders. Although the core symptoms of anxiety disorders (anxiety and worry) differ from the core symptoms of major depression (loss of interest and depressed mood), there is considerable overlap among the rest of the symptoms associated with these disorders (compare the “anxiety disorders” puzzle on the right to the “MDD” puzzle on the left). For example, fatigue, sleep difficulties, and problems concentrating are common to both types of disorders.
Anxiety disorders have considerable symptom overlap with major depression (see those symptoms surrounding core features shown in Figure 9-1), particularly sleep disturbance, problems concentrating, fatigue, and psychomotor/arousal symptoms. Each anxiety disorder also has a great deal of symptom overlap with other anxiety disorders (Figures 9-2 through 9-5). Anxiety disorders are also extensively comorbid, not only with major depression, but also with each other, since many patients qualify over time for a second or even third concomitant anxiety disorder. Finally, anxiety disorders are frequently comorbid with many other conditions such as substance abuse, attention deficit hyperactivity disorder, bipolar disorder, pain disorders, sleep disorders, and more.
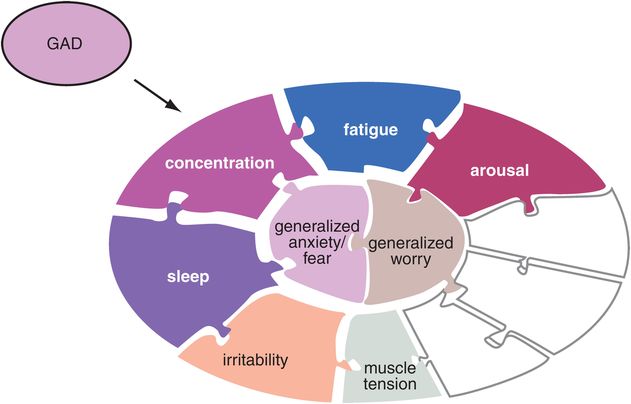
Figure 9-2. Generalized anxiety disorder (GAD). The symptoms typically associated with GAD are shown here. These include the core symptoms of generalized anxiety and worry as well as increased arousal, fatigue, difficulty concentrating, sleep problems, irritability, and muscle tension. Many of these symptoms, including the core symptoms, are present in other anxiety disorders as well.
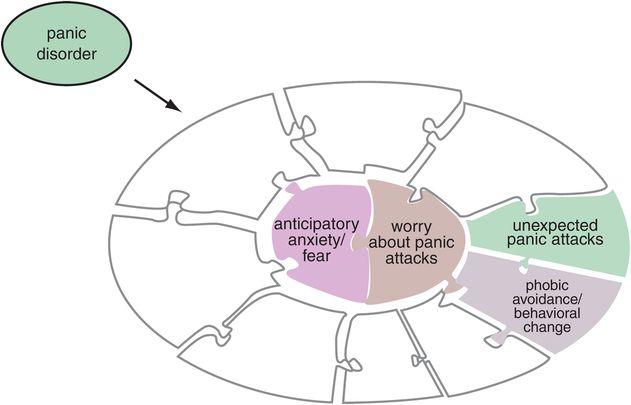
Figure 9-3. Panic disorder. The characteristic symptoms of panic disorder are shown here, with core symptoms of anticipatory anxiety as well as worry about panic attacks. Associated symptoms are the unexpected panic attacks themselves and phobic avoidance or other behavioral changes associated with concern over panic attacks.
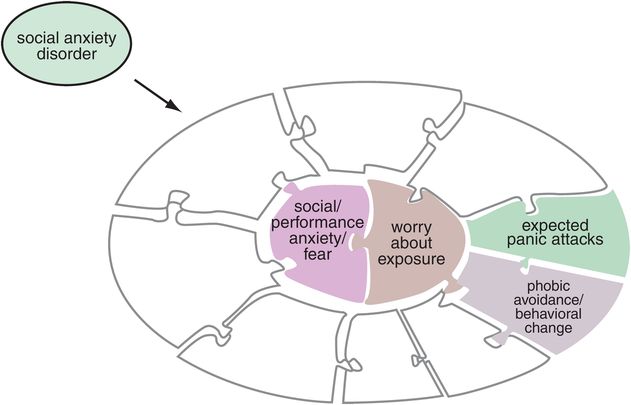
Figure 9-4. Social anxiety disorder. Symptoms of social anxiety disorder, shown here, include the core symptoms anxiety or fear over social performance plus worry about social exposure. Associated symptoms are panic attacks that are predictable and expected in certain social situations as well as phobic avoidance of those situations.

Figure 9-5. Posttraumatic stress disorder (PTSD). The characteristic symptoms of PTSD are shown here. These include the core symptoms of anxiety while the traumatic event is being re-experienced as well as worry about having the other symptoms of PTSD, such as increased arousal and startle responses, sleep difficulties including nightmares, and avoidance behaviors.
So, what is an anxiety disorder? These disorders all seem to maintain the core features of some form of anxiety or fear coupled with some form of worry, but their natural history over time shows them to morph from one into another, to evolve into full syndrome expression of anxiety disorder symptoms (Figure 9-1) and then to recede into subsyndromal levels of symptoms only to reappear again as the original anxiety disorder, a different anxiety disorder (Figures 9-2 through 9-5), or major depression (Figure 9-1). If anxiety disorders all share core symptoms of fear and worry (Figures 9-1 and 9-6) and, as we shall see later in this chapter, are all basically treated with the same drugs, including many of the same drugs that treat major depression, the question now arises, what is the difference between one anxiety disorder and another? Also, one could ask, what is the difference between major depression and anxiety disorders? Are all these entities really different disorders, or are they instead different aspects of the same illness?

Figure 9-6. Anxiety: the phenotype. Anxiety can be deconstructed, or broken down, into the two core symptoms of fear and worry. These symptoms are present in all anxiety disorders, although what triggers them may differ from one disorder to the next.
Overlapping symptoms of major depression and anxiety disorders
Although the core symptoms of major depression (depressed mood or loss of interest) differ from the core symptoms of anxiety disorders (fear and worry), there is a great deal of overlap with the other symptoms considered diagnostic both for a major depressive episode and for several different anxiety disorders (Figure 9-1). These overlapping symptoms include problems with sleep, concentration, and fatigue as well as psychomotor/arousal symptoms (Figure 9-1). It is thus easy to see how the gain or loss of just a few additional symptoms can morph a major depressive episode into an anxiety disorder (Figure 9-1) or one anxiety disorder into another (Figures 9-2 through 9-5).
From a therapeutic point of view, it may matter little what the specific diagnosis is across this spectrum of disorders (Figures 9-1 through 9-5). That is, first-line psychopharmacological treatments may not be much different for a patient who currently qualifies for a major depressive episode plus the symptom of anxiety (but not an anxiety disorder) versus a patient who currently qualifies for a major depressive episode plus a comorbid anxiety disorder with full criteria anxiety symptoms. Although it can be useful to make specific diagnoses for following patients over time and for documenting the evolution of symptoms, the emphasis from a psychopharmacological point of view is increasingly to take a symptom-based therapeutic strategy to patients with any of these disorders because the brain is not organized according to the DSM, but according to brain circuits with topographical localization of function. That is, specific treatments can be tailored to the individual patient by deconstructing whatever disorder the patient has into a list of the specific symptoms a given patient is experiencing (see Figures 9-2 through 9-5), and then matching these symptoms to hypothetically malfunctioning brain circuits regulated by specific neurotransmitters in order to rationally select and combine psychopharmacological treatments to eliminate all symptoms and get the patient to remission.
Overlapping symptoms of different anxiety disorders
Although there are different diagnostic criteria for different anxiety disorders (Figures 9-2 though 9-5), these are constantly changing, and many do not even consider obsessive–compulsive disorder to be an anxiety disorder any longer (OCD is discussed in Chapter 14 on impulsivity). All anxiety disorders have overlapping symptoms of anxiety/fear coupled with worry (Figure 9-6). Remarkable progress has been made in understanding the circuitry underlying the core symptom of anxiety/fear based upon an explosion of neurobiological research on the amygdala (Figures 9-7 through 9-14). The links between the amygdala, fear circuits, and treatments for the symptom of anxiety/fear across the spectrum of anxiety disorders are discussed throughout the rest of this chapter.
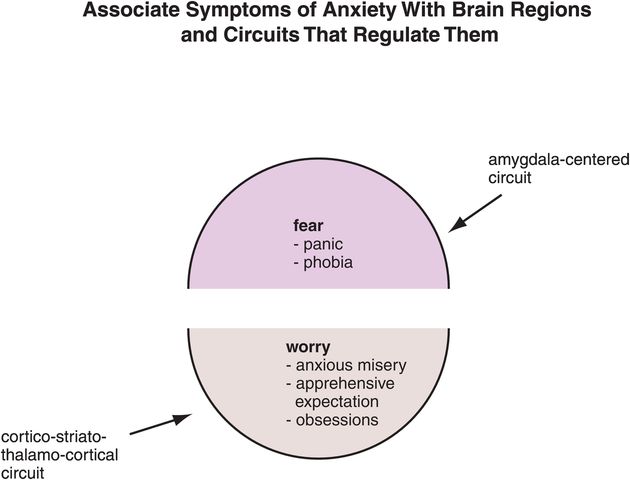
Figure 9-7. Linking anxiety symptoms to circuits. Anxiety and fear symptoms (e.g., panic, phobias) are regulated by an amygdala-centered circuit. Worry, on the other hand, is regulated by a cortico-striato-thalamo-cortical (CSTC) loop. These circuits may be involved in all anxiety disorders, with the different phenotypes reflecting not unique circuitry but rather divergent malfunctioning within those circuits.
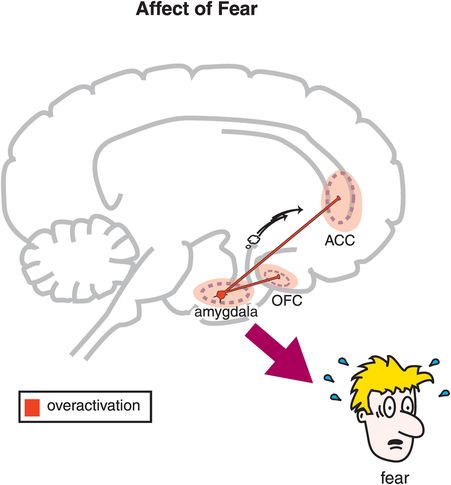
Figure 9-8. Affect of fear. Feelings of fear are regulated by reciprocal connections between the amygdala and the anterior cingulate cortex (ACC) and the amygdala and the orbitofrontal cortex (OFC). Specifically, it may be that overactivation of these circuits produces feelings of fear.

Figure 9-9. Avoidance. Feelings of fear may be expressed through behaviors such as avoidance, which is partly regulated by reciprocal connections between the amygdala and the periaqueductal gray (PAG). Avoidance in this sense is a motor response and may be analogous to freezing under threat. Other motor responses are to fight or to run away (flight) in order to survive threats from the environment.

Figure 9-10. Endocrine output of fear. The fear response may be characterized in part by endocrine effects such as increases in cortisol, which occur because of amygdala activation of the hypothalamic–pituitary–adrenal (HPA) axis. Prolonged HPA activation and cortisol release can have significant health implications, such as increased risk of coronary artery disease, type 2 diabetes, and stroke.

Figure 9-11. Breathing output. Changes in respiration may occur during a fear response; these changes are regulated by activation of the parabrachial nucleus (PBN) via the amygdala. Inappropriate or excessive activation of the PBN can lead not only to increases in the rate of respiration but also to symptoms such as shortness of breath, exacerbation of asthma, or a sense of being smothered.
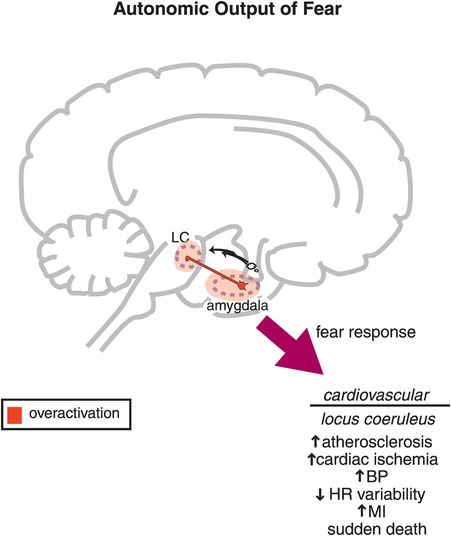
Figure 9-12. Autonomic output of fear. Autonomic responses are typically associated with feelings of fear. These include increases in heart rate (HR) and blood pressure (BP), which are regulated by reciprocal connections between the amygdala and the locus coeruleus (LC). Long-term activation of this circuit may lead to increased risk of atherosclerosis, cardiac ischemia, change in BP, decreased HR variability, myocardial infarction (MI), or even sudden death.
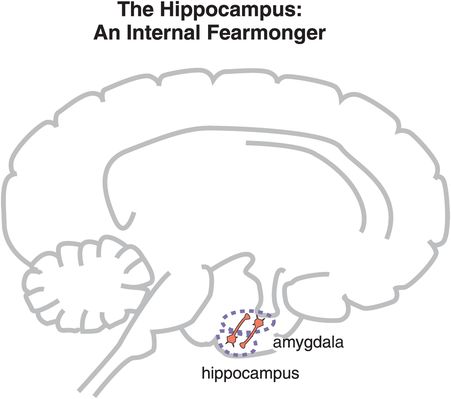
Figure 9-13. The hippocampus and re-experiencing. Anxiety can be triggered not only by an external stimulus but also by an individual’s memories. Traumatic memories stored in the hippocampus can activate the amygdala, causing the amygdala, in turn, to activate other brain regions and generate a fear response. This is termed re-experiencing, and it is a particular feature of posttraumatic stress disorder.
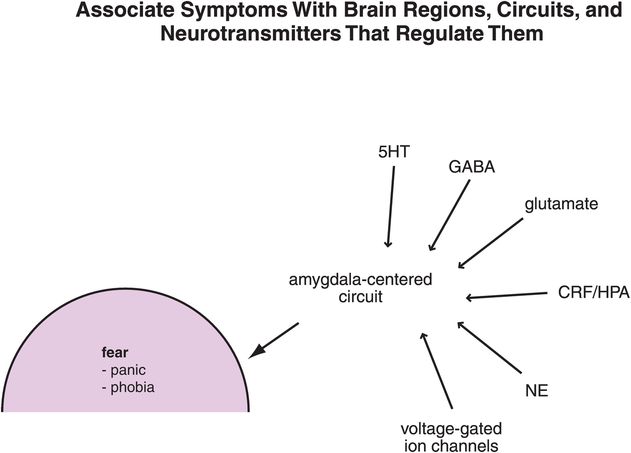
Figure 9-14. Linking anxiety symptoms to circuits to neurotransmitters. Symptoms of anxiety/fear are associated with malfunctioning of amygdala-centered circuits; the neurotransmitters that regulate these circuits include serotonin (5HT), γ-aminobutyric acid (GABA), glutamate, corticotropin-releasing factor (CRF), and norepinephrine (NE), among others. In addition, voltage-gated ion channels are involved in neurotransmission within these circuits.
Worry is the second core symptom shared across the spectrum of anxiety disorders (Figure 9-7). This symptom is hypothetically linked to the functioning of cortico-striato-thalamo-cortical (CSTC) loops. The links between the CSTC circuits, “worry loops,” and treatments for the symptom of worry across the spectrum of anxiety disorders are discussed later in this chapter (see also Figures 9-15 through 9-17, 9-26, and 9-29). We shall see that what differentiates one anxiety disorder from another may not be the anatomical localization, or the neurotransmitters regulating fear and worry in each of these disorders (Figures 9-6 and 9-7), but the specific nature of malfunctioning within these same circuits in various anxiety disorders. That is, in generalized anxiety disorder (GAD), malfunctioning in the amygdala and CSTC worry loops may be hypothetically persistent, and unremitting, yet not severe (Figure 9-2), whereas malfunctioning may be theoretically intermittent but catastrophic in an unexpected manner for panic disorder (Figure 9-3) or in an expected manner for social anxiety (Figure 9-4). Circuit malfunctioning may be traumatic in origin and conditioned in posttraumatic stress disorder (PTSD: Figure 9-5).
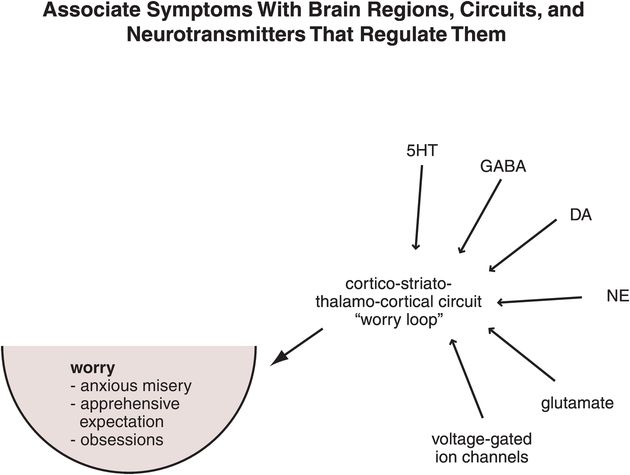
Figure 9-15. Linking worry symptoms to circuits to neurotransmitters. Symptoms of worry are associated with malfunctioning of cortico-striato-thalamo-cortical (CSTC) loops, which are regulated by serotonin (5HT), γ-aminobutyric acid (GABA), dopamine (DA), norepinephrine (NE), glutamate, and voltage-gated ion channels.

Figure 9-16. Worry/obsessions circuit. Shown here is a cortico-striato-thalamo-cortical (CSTC) loop originating and ending in the dorsolateral prefrontal cortex (DLPFC). Overactivation of this circuit may lead to worry or obsessions.
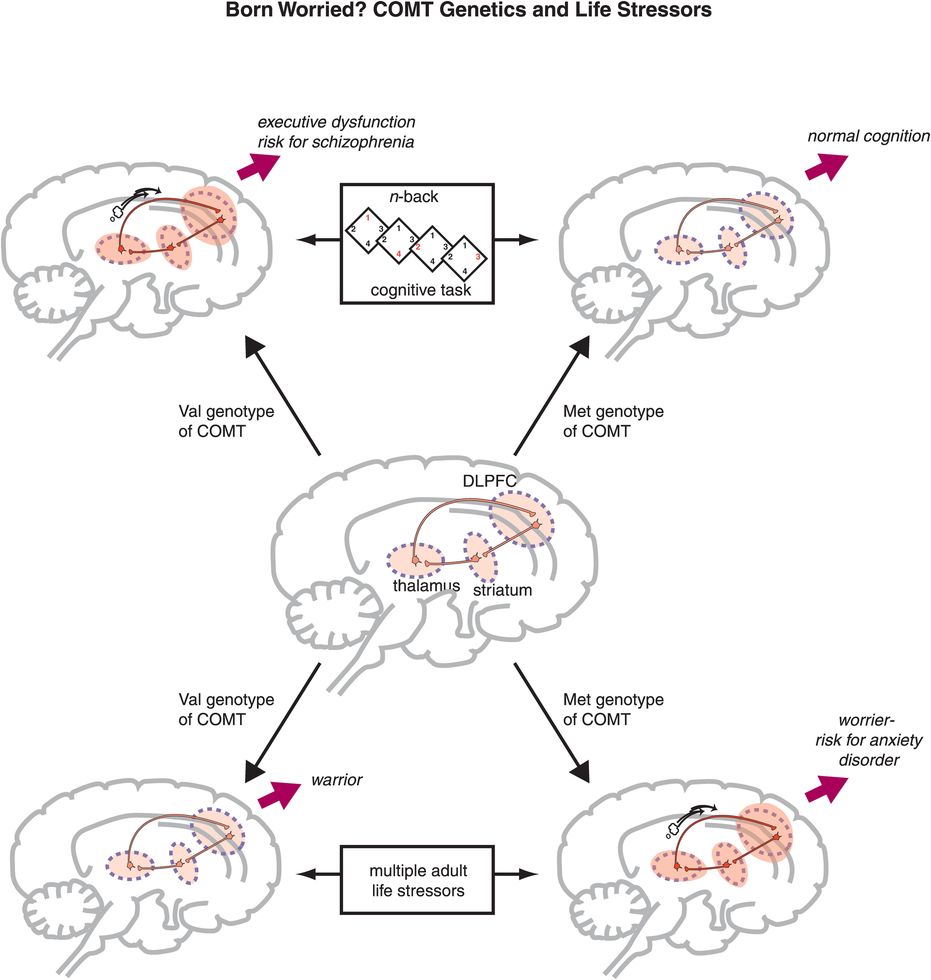
Figure 9-17. COMT genetics and life stressors. Activity in the cortico-striato-thalamo-cortical (CSTC) loop may vary during cognitive tasks depending on the variant of catechol-O-methyl-transferase (COMT) that an individual has (upper portion of figure). Thus, those with the Met genotype for COMT (i.e., those who have lower COMT activity and thus higher dopamine levels) may have “normal” activation and no problems with performance during a cognitive task, whereas those with the Val genotype may exhibit inefficiency of cognitive information processing, require overactivation of this circuit, and potentially make more errors during the same task. These latter individuals may also be at increased risk for schizophrenia. Similarly, the variant of COMT that an individual has may affect response to stress, since the CSTC loop also regulates worry. In this case, however, the beneficial genotype may be reversed. That is, because individuals with the Met genotype have lower COMT activity and thus higher dopamine levels, dopamine release in response to stress may be excessive and contribute to worry and risk for anxiety disorders. Those with the Val genotype, on the other hand, may be less reactive to stress because COMT can destroy the excess dopamine.
The amygdala and the neurobiology of fear
The amygdala, an almond-shaped brain center located near the hippocampus, has important anatomical connections that allow it to integrate sensory and cognitive information and then determine whether there will be a fear response. Specifically, the affect or feeling of fear may be regulated via reciprocal connections that the amygdala shares with key areas of prefrontal cortex that regulate emotions, namely the orbitofrontal cortex and the anterior cingulate cortex (Figure 9-8). However, fear is not just a feeling. The fear response can also include motor responses. Depending upon the circumstances and one’s temperament, those motor responses could be fight, flight, or freezing in place. Motor responses of fear are regulated in part by connections between the amygdala and the periaqueductal gray area of the brainstem (Figure 9-9).
There are also endocrine reactions that accompany fear, in part due to connections between the amygdala and the hypothalamus, causing changes in the HPA (hypothalamic–pituitary–adrenal) axis, and thus of cortisol levels. A quick boost of cortisol may enhance survival when encountering a real but short-term threat. However, chronic and persistent activation of this aspect of the fear response can lead to increased medical comorbidity, including increased rates of coronary artery disease, type 2 diabetes, and stroke (Figure 9-10), and potentially to hippocampal atrophy as well (discussed in Chapter 6 and shown in Figure 6-39B). Breathing can also change during a fear response, regulated in part by the connections between amygdala and the parabrachial nucleus in the brainstem (Figure 9-11). An adaptive response to fear is to accelerate respiratory rate when having a fight/flight reaction to enhance survival, but in excess this can lead to unwanted symptoms of shortness of breath, exacerbation of asthma, or a false sense of being smothered (Figure 9-11), all symptoms common during anxiety, and especially during attacks of anxiety such as panic attacks.
The autonomic nervous system is attuned to fear, and is able to trigger responses from the cardiovascular system such as increased pulse and blood pressure for fight/flight reactions and survival during real threats. These autonomic and cardiovascular responses are mediated by connections between the amygdala and the locus coeruleus, home of the noradrenergic cell bodies (Figure 9-12; noradrenergic neurons are discussed in Chapter 6, and noradrenergic pathways and neurons are illustrated in Figures 6-25 through 6-30, and also Figure 6-32). When autonomic responses are repetitive and inappropriately or chronically triggered as part of an anxiety disorder, this can lead to increases in atherosclerosis, cardiac ischemia, hypertension, myocardial infarction, and even sudden death (Figure 9-12). “Scared to death” may not always be an exaggeration or a figure of speech! Finally, anxiety can be triggered internally from traumatic memories stored in the hippocampus and activated by connections with the amygdala (Figure 9-13), especially in conditions such as posttraumatic stress disorder.
The processing of the fear response is regulated by the numerous neuronal connections flowing into and out of the amygdala. Each connection utilizes specific neurotransmitters acting at specific receptors (Figure 9-14). What is known about these connections is that not only are several neurotransmitters involved in the production of symptoms of anxiety at the level of the amygdala, but numerous anxiolytic drugs have actions upon these specific neurotransmitter systems to relieve the symptoms of anxiety and fear (Figure 9-14). The neurobiological regulators of the amygdala, including the neurotransmitters GABA, 5HT, and NE, the voltage-gated calcium channels, and anxiolytics that act upon these neurotransmitters in order to mediate their therapeutic actions, are specifically discussed later in this chapter.
Cortico-striato-thalamo-cortical (CSTC) loops and the neurobiology of worry
Dopamine and born worried?
The second core symptom of anxiety disorders, worry, involves another unique circuit (Figure 9-15). Worry, which can include anxious misery, apprehensive expectations, catastrophic thinking, and obsessions, is linked to cortico-striato-thalamo-cortical (CSTC) feedback loops from the prefrontal cortex (Figures 9-15 and 9-16). Some experts theorize that similar CSTC feedback loops regulate the related symptoms of ruminations, obsessions, and delusions, all of these symptoms being types of recurrent thoughts. Several neurotransmitters and regulators modulate these circuits, including serotonin, GABA, dopamine, norepinephrine, glutamate, and voltage-gated ion channels (Figure 9-15). These overlap greatly with many of the same neurotransmitters and regulators that modulate the amygdala (Figure 9-14). Since various genotypes for the enzyme COMT (catechol-O-methyl-transferase) regulate the availability of one of these neurotransmitters, namely dopamine, in the prefrontal cortex, differences in dopamine availability may impact the risk for worry and anxiety disorder and help to determine whether you are “born worried” and vulnerable to developing an anxiety disorder, particularly under stress (Figure 9-17).
Warriors versus worriers
In Chapter 4 the impact of genetic variants of COMT on cognitive functioning are mentioned in relation to schizophrenia. Specifically, normal controls with the Met variant of COMT have more efficient information processing in the dorsolateral prefrontal cortex (DLPFC) during a cognitive task such as the n-back test. These subjects have lower COMT activity, higher dopamine levels, and presumably better information processing during tasks of executive functioning that recruit circuits in the DLPFC. Because of more efficient cognitive information processing, such subjects also have a lower risk for schizophrenia than subjects who are Val carriers of COMT (Figure 4-44).
At first glance, it would seem that all the biological advantages go to those with the Met variant of COMT. However, that is not necessarily true when it comes to processing stressors that cause dopamine release. With the Met genotype and its low COMT activity and high dopamine levels, stressors can hypothetically produce too much dopamine activity, which actually decreases the efficiency of information processing under stress and creates the symptoms of anxiety and worry (“worriers”). Under stress, therefore, it appears that Val carriers of COMT with their higher enzyme activity and lower dopamine levels are hypothetically able to handle the increased dopamine release that comes with stress by optimizing their information processing; thus they are “warriors” who are not afraid or worried when stressed. Dopamine is just one of the potential regulators of worry circuits and CSTC loops.
GABA and benzodiazepines
GABA (γ-aminobutyric acid) is one of the key neurotransmitters involved in anxiety and in the anxiolytic action of many drugs used to treat the spectrum of anxiety disorders. GABA is the principal inhibitory neurotransmitter in the brain and normally plays an important regulatory role in reducing the activity of many neurons, including those in the amygdala and those in CSTC loops. Benzodiazepines, perhaps the best-known and most widely used anxiolytics, act by enhancing GABA actions at the level of the amygdala and at the level of the prefrontal cortex within CSTC loops to relieve anxiety. To understand how GABA regulates brain circuits in anxiety, and to understand how benzodiazepines exert their anxiolytic actions, it is important to understand the GABA neurotransmitter system, including how GABA is synthesized, how GABA action is terminated at the synapse, and the properties of GABA receptors (Figures 9-18 through 9-24).
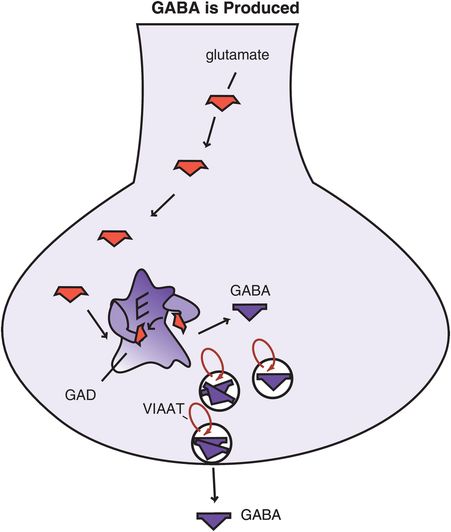
Figure 9-18. Gamma-aminobutyric acid (GABA) is produced. The amino acid glutamate, a precursor to GABA, is converted to GABA by the enzyme glutamic acid decarboxylase (GAD). After synthesis, GABA is transported into synaptic vesicles via vesicular inhibitory amino acid transporters (VIAATs) and stored until its release into the synapse during neurotransmission.
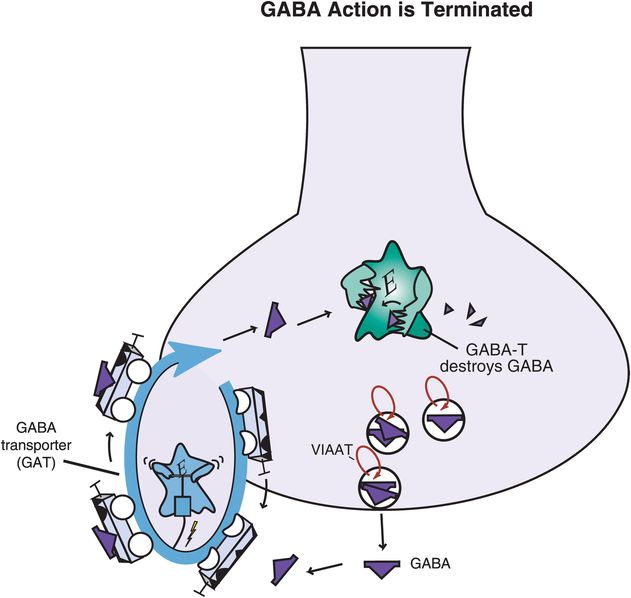
Figure 9-19. Gamma-aminobutyric acid (GABA) action is terminated. GABA’s action can be terminated through multiple mechanisms. GABA can be transported out of the synaptic cleft and back into the presynaptic neuron via the GABA transporter (GAT), where it may be repackaged for future use. Alternatively, once GABA has been transported back into the cell, it may be converted into an inactive substance via the enzyme GABA transaminase (GABA-T).
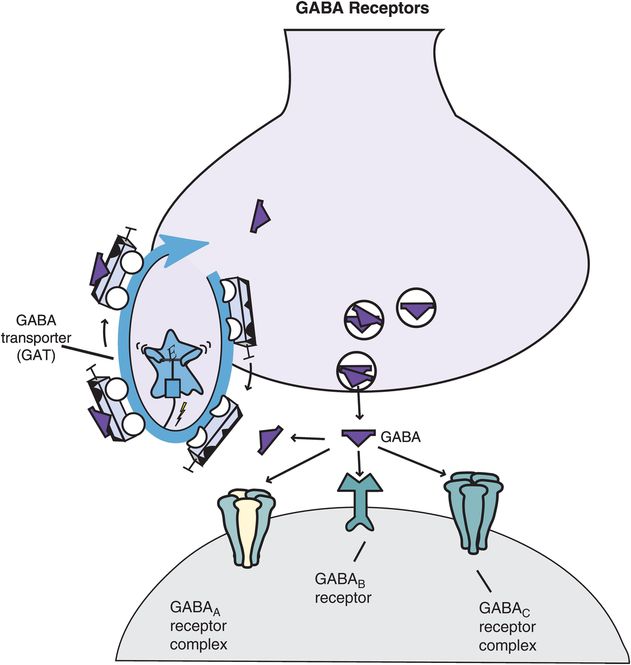
Figure 9-20. Gamma-aminobutyric acid (GABA) receptors. Shown here are receptors for GABA that regulate its neurotransmission. These include the GABA transporter (GAT) as well as three major types of postsynaptic GABA receptors: GABAA, GABAB, and GABAC. GABAA and GABAC receptors are ligand-gated ion channels; they are part of a macromolecular complex that forms an inhibitory chloride channel. GABAB receptors are G-protein-linked receptors that may be coupled with calcium or potassium channels.
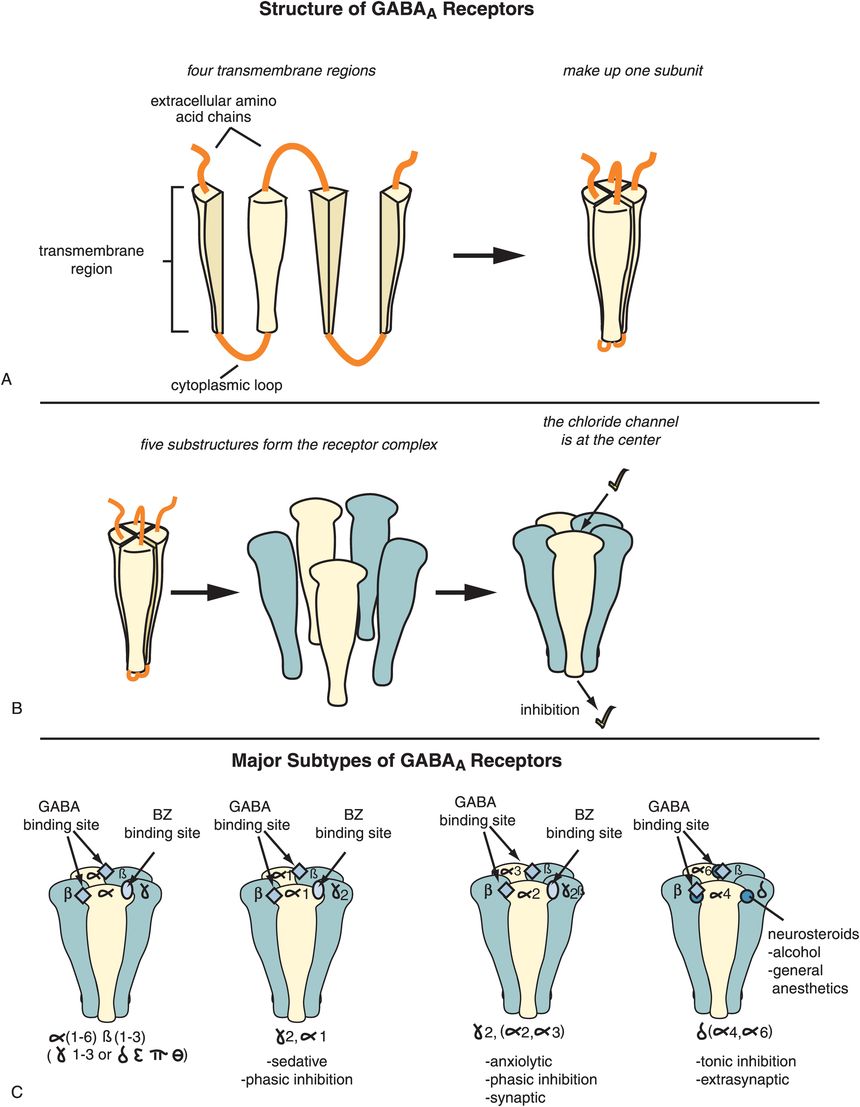
Figure 9-21. Gamma-aminobutyric acid-A (GABAA) receptors. (A) Shown here are the four transmembrane regions that make up one subunit of a GABAA receptor. (B) There are five copies of these subunits in a fully constituted GABAA receptor, at the center of which is a chloride channel. (C) Different types of subunits (also called isoforms or subtypes) can combine to form a GABAA receptor. These include six different alpha (α) isoforms, three different beta (β) isoforms, three different gamma (γ) isoforms, delta (δ), epsilon (ε), pi (π), theta (θ), and three different rho (ρ) isoforms. The ultimate type and function of each GABAA receptor subtype will depend on which subunits it contains. Benzodiazepine-sensitive GABAA receptors (middle two) contain γ and α1–3 subunits and mediate phasic inhibition triggered by peak concentrations of synaptically released GABA. Benzodiazepine-sensitive GABAA receptors containing α1 subunits are involved in sleep (second from left), while those that contain α2 and/or α3 subunits are involved in anxiety (second from right). GABAA receptors containing α4, α6, γ1, or δ subunits (far right) are benzodiazepine-insensitive, are located extrasynaptically, and regulate tonic inhibition.
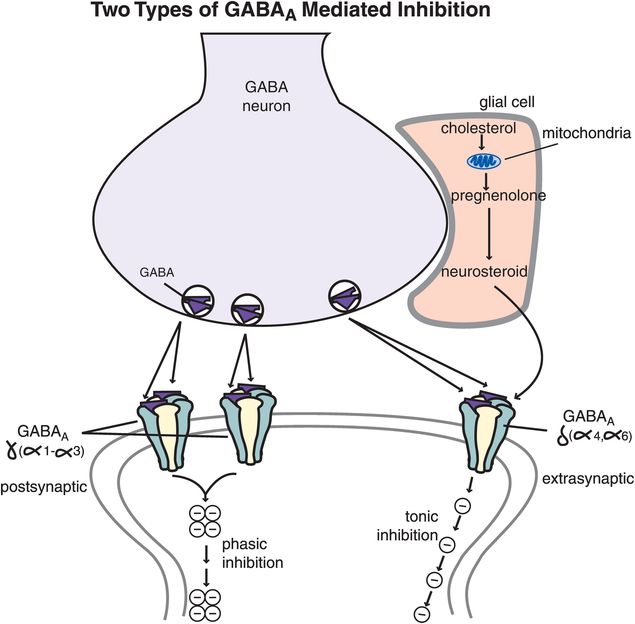
Figure 9-22. GABAA mediation of tonic and phasic inhibition. Benzodiazepine-sensitive GABAA receptors (those that contain γ and α1–3 subunits) are postsynaptic receptors that mediate phasic inhibition, which occurs in bursts triggered by peak concentrations of synaptically released GABA. Benzodiazepine-insensitive GABAA receptors (those containing α4, α6, γ1, or δ subunits) are extrasynaptic and capture GABA that diffuses away from the synapse as well as neurosteroids that are synthesized and released by glia. These receptors mediate inhibition that is tonic (i.e., mediated by ambient levels of extracellular GABA that has escaped from the synapse).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


