FIGURE 35-1 Pulmonary vein (PV ) ectopy initiating atrial fibrillation (AF). Surface leads V1, II, and III, recording from inferior left (LI) PV, coronary sinus (CS), and ablation catheter (ABL). A premature beat (arrow) arising from the LIPV, earliest LA 10-1, precedes atrial activity on the surface ECG (arrow on surface ECG), conducts to the left atrium (LA) and initiates AF.
ELECTROPHYSIOLOGICAL PROPERTIES OF PULMONARY VEINS
Experimental evidence from isolated, blood-perfused dog hearts support triggered activity, reentry, and automaticity as the mechanisms of arrhythmogenesis in PVs.8 Nonhomogenous muscle fiber orientation at the ostia of PVs correlates with zones of delayed conduction and fractionated signals facilitating reentry within the PVs. These electrophysiological properties of PVs were confirmed with optical mapping in atrial preparations of dog hearts demonstrating delayed conduction, heterogeneous depolarization, conduction block, and reentry in 60% of preparations. Besides, sustained focal discharges localized near the PV ostia were also demonstrated with isoproterenol infusion.9
In clinical studies, the effective refractory period (ERP) of the four PVs was shorter than the LA ERP in patients with AF. In the same study, the opposite was true for patients without AF. Decremental conduction between the PV and LA and the slow conduction within the PVs was also noted to be more prevalent in patients with AF suggesting a pivotal role for reentry within PV musculature in AF perpetuation.10 Furthermore, pace-termination and entrainment of PV-induced arrhythmias provide indirect evidence supportive of reentry as a mechanism for AF.
There is also evidence that automaticity and triggered activity may play a role in the genesis of AF. Discrete ectopic discharges and adenosine sensitive focal tachycardias have been demonstrated using high-density mapping within PVs, and there was no evidence of reentry in some reports. Besides, early reports in animal models have identified pacemaker regions in the distal parts of the muscular sleeves within the pulmonary vein where it joins the smooth muscle. Those PV sites were referred to as subsidiary pacemakers in the 1980s and are capable of developing oscillatory after-potentials, large enough to reach threshold and cause extrasystoles.11 Digitalis treated atrial preparations were shown to develop oscillatory after-potentials and act as ectopic foci.
PULMONARY VEIN ANATOMY
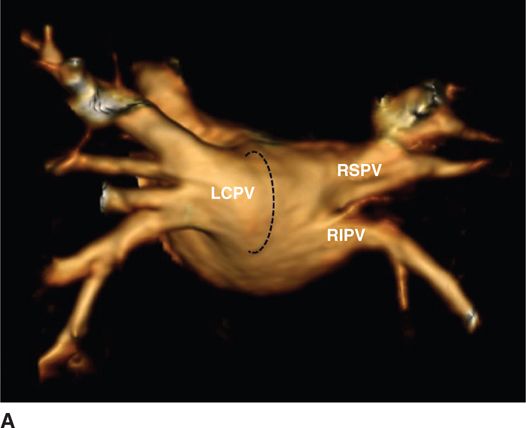
Embryologically, the PVs arise from the posterior LA without distinct boundaries separating the ostium, antrum, and LA (Figure 35-2). Nonuniform sleeves of myocardium extending from the LA into PVs constitute the arrhythmogenic substrate for paroxysmal AF. These myocardial sleeves are more developed in the upper than the lower PVs, extend between 2 and 25 mm distally into the vein, and are thickest at the LA-PV junction. Sleeves tend to be thicker in the “carina” region separating the inferior from the superior veins with fibrous tissue filling gaps between those sleeves at the LA-PV junction and within the PVs. Sleeves most commonly wrap around the LA-PV junction in a circumferential manner, but some are oriented in a longitudinal or oblique fashion. Therefore, the anisotropic arrangement of these muscle fibers may provide a substrate for reentry.
FIGURE 35-2A Magnetic resonance imaging reconstruction of the atrial and pulmonary vein anatomy. (A) Three-dimensional reconstruction of the left atrium and pulmonary veins, view from a posterior perspective. Note the left common ostium (LCPV) of the left pulmonary veins and the separate ostia of the right inferior pulmonary vein (RIPV) and right superior pulmonary vein (RSPV).
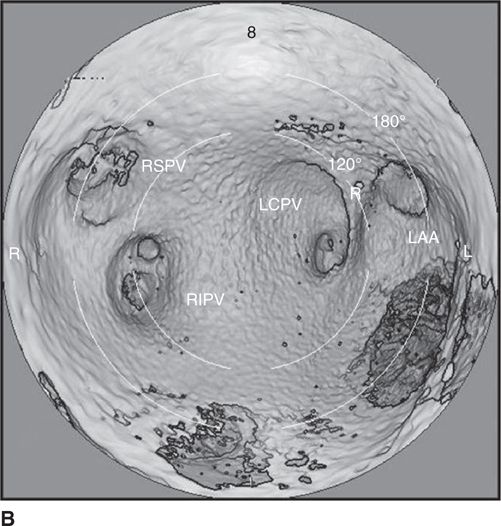
FIGURE 35-2B Computed tomography reconstruction of the atrial and pulmonary vein (PV) anatomy. Intra-atrial reconstructed views of the posterior LA. Note the “saddles” between adjacent PVs. The complex, funnel-shaped approaches to the veins are apparent. In this case, there is a common ostium for the left-sided veins (LCPV). Also demonstrated is the narrow ridge that separates the left PVs from the left atrial appendage (LAA). Abbreviations: R, ridge between LAA and PVs; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
CATHETER ABLATION OF ATRIAL FIBRILLATION
According to current guidelines, catheter ablation for symptomatic AF is considered a second-line treatment after failure of antiarrhythmic medications. Expert consensus acknowledges the pivotal role of PVs in AF and recommends complete electrical isolation of PVs.12 Electrical isolation requires demonstration of entrance block into the vein. PV isolation is often sufficient in patients with paroxysmal AF. However, in patients with persistent AF who may have electroanatomical remodeling, modification of the atrial substrate beyond PVs may often be necessary.13
Initial efforts targeted arrhythmogenic foci within the PVs by focal applications of radiofrequency energy often within the PV or one of its branches.5 However, this approach was associated with a high recurrence rate both from the same PV and also other PVs and with a high incidence of PV stenosis.14 Since the electrical connection between the PVs and the left atrium occur via insulated, discrete myocardial fibers, referred to as PV fascicles, complete isolation of PVs by segmental ostial ablation followed.5,15 Subsequently, left atrial circumferential ablation or wide area circumferential ablation (Figure 35-3) that included most if not all of the antrum was shown to be superior to isolation of PVs at the ostial level.15
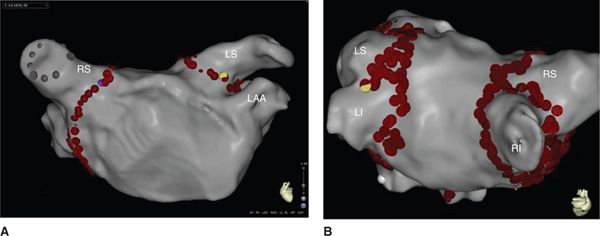
FIGURE 35-3 Three-dimensional reconstruction of the left atrium using the Biosense CARTO 3 mapping system. (A) Anteroposterior (AP) view of the left atrium (LA) and pulmonary veins (PVs) with circumferential ablation (red dots) around the antrum. (B) Posteroanterior (PA) view with a rightward tilt of the left atrium (LA) and pulmonary veins (PVs) with circumferential ablation (red dots) around the antra of individual PVs. Abbreviations: LAA, left atrial appendage; LI, left inferior pulmonary vein; LS, left superior pulmonary vein; RI, right inferior pulmonary vein; RS, right superior pulmonary vein.
The PV antrum that includes most of the posterior left atrium shares similar histoembryological origin to the tubular portion of the PV and appears to have a similar arrhythmogenic potential as the PVs. As a matter of fact a majority of the non-PV foci that initiate AF arise from the posterior left atrium and PV antrum. Later ablation strategies evolved as antral PV isolation that aims to isolate the PVs by antral ablation specifically targeting antral potentials (that have similar characteristics to the PV potentials such as high frequency activation) and by confirming complete isolation of PVs.12 The mechanisms of superior efficacy of antral PV isolation may include: (1) elimination of PV arrhythmogenicity; (2) elimination of drivers of AF such as rotors that often have anchor points in the antrum of PVs; (3) elimination of non-PV triggers that may originate within the PV antrum; (4) debulking of the left atrium; and (5) ablation of ganglionated plexi that often are located on the epicardial aspect of the PV antrum.
Technique
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree