Antiviral Drug Nephrotoxicity
Shane M. Meehan, MBBCh
Key Facts
Terminology
Kidney dysfunction due to exposure to antiviral agents
Etiology/Pathogenesis
Direct tubular toxicity: Tenofovir, adefovir, acyclovir, cidofovir
Tubulointerstitial nephritis (TIN): Indinavir, atazanavir, abacavir, efavirenz, foscarnet
Crystal nephropathy: Acyclovir, indinavir
Glomerulopathy: Foscarnet, valacyclovir, acyclovir, enfuvirtide
Microscopic Pathology
Acute tubular injury or necrosis ± TIN
Karyomegaly + megamitochondria
Proximal tubules are most severely affected
Interstitial fibrosis may be prominent
Crystal nephropathy: Intrarenal precipitation of crystallized salts of antiviral agent (indinavir and foscarnet)
TIN: Acute and chronic
Glomerular crystals (foscarnet), thrombotic microangiopathy (acyclovir)
Ancillary Tests
Mitochondrial enlargement, dysmorphism, distortion of cristae; mitochondrial depletion on electron microscopy
Infrared spectroscopy allows definitive identification of crystal composition
TERMINOLOGY
Synonyms
Antiviral nephrotoxicity
Definitions
Kidney dysfunction due to exposure to antiviral agents
ETIOLOGY/PATHOGENESIS
Environmental Exposure
Antiviral agents with known nephrotoxicity
Nucleoside analogs (reverse transcriptase inhibitors): Acyclovir, valacyclovir, ganciclovir, abacavir, lamivudine, didanosine
Nucleotide analogs (reverse transcriptase inhibitors): Tenofovir, adefovir, cidofovir
Peptide analogs (protease inhibitors): Indinavir, ritonavir, atazanavir
Pyrophosphate analogs: Foscarnet (trisodium phosphonoformate)
Fusion or entry inhibitors: Enfurtivide
Other agents: Intravenous immunoglobulin, interferon-α
Risk factors for nephrotoxicity
Kidney
Drugs are concentrated in specific nephron segments; proximal tubules are at greatest risk of injury
Organic anion transporter system concentrates tenofovir and cidofovir in proximal tubules
Patient
Infection, e.g., HIV
Underlying chronic kidney disease and dehydration
Pharmacogenetics and immune response genes
Drug
Dose dependent: High dose increases risk of renal injury
Solubility: Concentration and pH dependent
Immune stimulatory potential
Pathogenesis
Direct tubular toxicity: Tenofovir, adefovir, acyclovir, cidofovir
Mitochondrial toxicity: Antiviral nucleoside and nucleotide analogs (ANA) act as competitive alternative substrate for mitochondrial thymidine kinase
ANA triphosphate inhibits mitochondrial DNA polymerase-γ, resulting in altered mitochondrial DNA
Mitochondrial depletion and mitochondrial DNA depletion are associated with tenofovir, adefovir, and cidofovir
Tubulointerstitial nephritis (TIN): Indinavir, atazanavir, abacavir, efavirenz, foscarnet
Dysfunction associated with inflammatory injury
Injury may be precipitated by tubular injury or idiosyncratic immunologic reactions ± effects of HIV infection
Crystal nephropathy: Acyclovir, indinavir
Crystals may be toxic to epithelium and may obstruct tubules
Crystal precipitation often associated with tubulointerstitial nephritis
Glomerulopathy: Foscarnet, valacyclovir, acyclovir, enfuvirtide
Glomerular crystal deposition
CLINICAL ISSUES
Presentation
Proximal tubulopathy and Fanconi syndrome: Cidofovir, tenofovir, adefovir, foscarnet, stavudine, lamivudine
Frequency: Tenofovir (0.3-2%), adefovir (up to 50% at high dose)
Distal tubular acidosis: Foscarnet
Nephrogenic diabetes insipidus: Foscarnet, didanosine, abacavir
Acute renal failure/kidney injury: Acyclovir, ganciclovir, cidofovir, indinavir, tenofovir, adefovir, foscarnet
Frequency: Tenofovir (0.5-1.5%), acyclovir (10%)
Crystalluria, lithiasis: Acyclovir, indinavir
Frequency: Indinavir (10-20%), acyclovir (12-48% with intravenous infusions), atazanavir (0.01%)
Crystalluria may be associated with acute kidney injury
Proteinuria: Cidofovir, foscarnet, interferon-α
Chronic renal failure: Cidofovir, indinavir, tenofovir
Treatment
Drug withdrawal or substitution
Hydration and restoration of high urinary output
Prognosis
Most acute dysfunction due to antiviral agents is reversible
Most acute kidney injuries and acute crystal nephropathies are reversible
TIN ± crystal deposits are reversible if interstitial fibrosis is limited
Lesions have limited reversibility if there is extensive scarring on biopsy
Anecdotal reports of end-stage renal failure due to cidofovir and foscarnet toxicity
MICROSCOPIC PATHOLOGY
Histologic Features
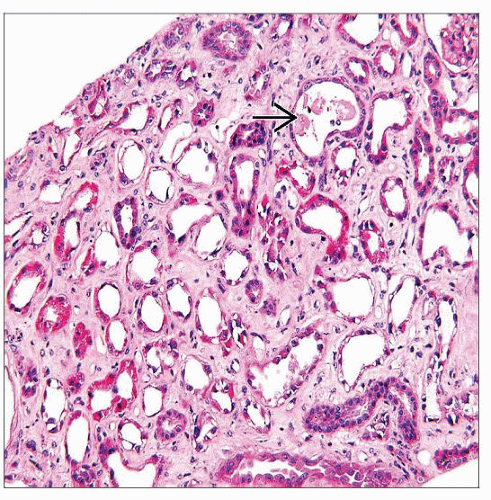
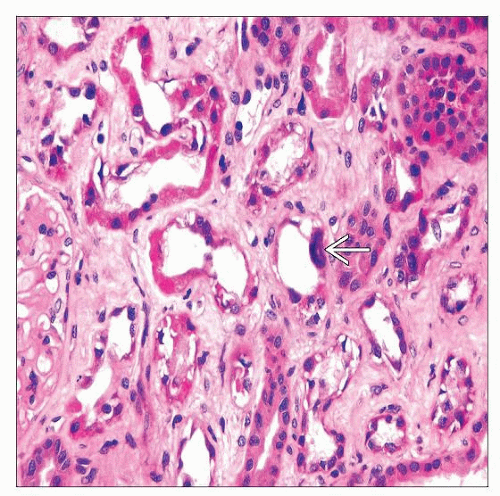
Acute tubular injury or necrosis
Tubular injury can be associated with most antiviral agents
Proximal tubules are most severely affected with loss of apical cytoplasm, brush border, irregular tubular luminal boundary
Karyomegaly with nuclear enlargement, irregular nuclear membrane, hyperchromatism, coarse nucleoli
Proximal tubular megamitochondria are rounded eosinophilic cytoplasmic inclusions; fuchsinophilic on trichrome stain; PAS negative
Megamitochondria are characteristic of tenofovir, but also described in cidofovir, adefovir, lamivudine, and stavudine toxicity
Distal tubular segments and collecting ducts also have evidence of injury with regeneration
Interstitial fibrosis may be prominent
Myoglobinuric acute tubular necrosis has been described with use of didanosine and zidovudine in patients with HIV infection
Intravenous immunoglobulin may be associated with osmotic tubulopathy
Crystal nephropathy: Intrarenal precipitation of crystallized salts of antiviral agent
Acyclovir
Distal tubular crystal deposits and mild tubulointerstitial inflammation without crystal deposits are both described
Foscarnet
Crystal deposits detectable in air-dried or alcohol-fixed frozen sections
Rhomboid and clustered polyhedral yellow crystals; washed out by aqueous fixatives
Birefringent on polarization microscopy
Sites of proximal tubular crystal deposition have luminal macrophages, giant cells, and tubular injury, all with cytoplasmic crystal clefts
Similar-appearing crystal clefts are seen in glomerular capillaries, associated with fibrin deposition and, rarely, crescent formation
Crystals are von Kossa positive
Infrared spectroscopy reveals trisodium foscarnet, as well as sodium and calcium phosphate salts containing foscarnet
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree