http://evolve.elsevier.com/Edmunds/NP/

 Key drug. Another drug, rivastigmine (Exelon), has an FDA indication for Parkinson’s disease dementia.
Key drug. Another drug, rivastigmine (Exelon), has an FDA indication for Parkinson’s disease dementia.
Parkinson’s disease (PD) is the most common neurodegenerative disorder after Alzheimer’s disease. The prevalence and incidence rates of PD increase with age, and the incidence in men is significantly higher (1.5 times higher) than in women. PD is not curable; symptoms progress and worsen over time. A neuroprotective treatment that can slow or halt disease progression has not yet been established. PD treatment is complex, owing to the array of motor and nonmotor features combined with early- and late-treatment adverse effects. Treatment for patients with PD is highly individualized.
Therapeutic Overview
The neurotransmitters acetylcholine and dopamine and others in the neurons of the substantia nigra modulate movement. Damage to these neurons caused by amyloid results in excess acetylcholine and diminished dopamine in the basal ganglia. Replacement and regulation of the neurotransmitters with exogenous medications represents the hallmark of PD management; however, to improve symptoms and avoid side effects, these medications must be able to cross the blood-brain barrier without being metabolized in the periphery.
Synthesis and Metabolism of Dopamine in the Nerve Terminals
Tyrosine is converted to levodopa (L-dopa) by tyrosine hydroxylase. L-Dopa then is enzymatically decarboxylated by L-amino acid decarboxylase (L-AAD) to form dopamine. Dopamine is stored in synaptic vesicles until needed and then is released into the synapse, where it activates dopamine (D) receptors. The action of dopamine is terminated by reuptake into presynaptic vesicles or metabolism via MAO-B or COMT. This results in the formation of hydrogen peroxide, which then is metabolized to water by glutathione. If glutathione is deficient, or a surplus of hydrogen peroxide exists, hydroxyl free radicals are formed, causing lipid peroxidation and cell membrane damage.
Pathophysiology
Parkinsonism is a chronic, debilitating disease with no known cure. The goals of treatment are to relieve the symptoms of the disease and to help the patient maintain independence and mobility. By origin, PD is categorized as (1) primary or idiopathic, (2) secondary or acquired, (3) heritable, or (4) multisystem. For purposes of this text, only IPD is discussed. Practitioners are urged to consult differential diagnostic references to distinguish among the types of parkinsonism, including drug-induced cases.
PD results from a relative excess of cholinergic activity and a deficiency of dopaminergic activity in the basal ganglia. Lewy bodies (amyloid inclusion bodies) in the neurons of the pars compacta region of the substantia nigra are common in the pathophysiology of parkinsonism. Damage to these dopaminergic neurons causes loss of dopamine at their terminal projections in the caudate nucleus and putamen. Dopamine normally inhibits the action of acetylcholine in the striatum; therefore, decreased concentrations of dopamine caused by neuronal degeneration result in unopposed acetylcholine. This manifests as tremors in the patient. Clinical signs and symptoms of parkinsonism develop after approximately 80% of dopaminergic neurons are lost.
Some understanding of the origin of IPD was gained when several intravenous drug abusers developed PD after injecting a meperidine analog, methylphenyltetrahydropyridine (MPTP). The MPP ion that was formed after oxidative metabolism of MPTP by MAO-B was found to be neurotoxic to melanin-containing neurons in the substantia nigra. Inhibition of MAO-B by selegiline prevented the formation of MPP. Epidemiologic studies have shown an increased risk of developing PD with rural living and exposure to well water, pesticides, herbicides, and wood pulp mills. Discovery of a gene that could be responsible for a certain type of familial PD has brought hope for further research into the mechanism and prevention of the disease, perhaps through the use of stem cells. Stem cells represent only a very small portion of all of the neuroprotection trials and other work currently under way.
Disease Process
Dr. James Parkinson first described paralysis agitans, or shaking palsy, in 1817. Today, we know this condition as PD, Parkinson’s syndrome, or parkinsonism. Age at onset of signs and symptoms varies; however, most patients first experience symptoms between the ages of 50 and 69. In up to 30% of patients, symptoms occur before age 50. Patients present with one or more of four typical clinical symptoms: tremor at rest, bradykinesia, rigidity, and postural instability. Some patients with IPD may present with only a resting tremor of 4 to 7 cycles/sec as the principal symptom. These patients usually experience a slower progression of disease and little mental status change. Patients who present with postural instability and gait difficulty, however, often have a more rapid disease progression that includes dementia and bradykinesia. Early research suggests that NSAIDs may protect against the development of PD.
Assessment
PD is a slow, insidiously progressive neurodegenerative disorder. Early signs and symptoms include paresthesias, dystonia, depression, pain, anosmia, constipation, and numbness, which progress to the classic clinical features of tremor, bradykinesia, rigidity, and postural instability. Other typical problems include micrographia, hypophonia, shuffling or festinating gait, lack of facial expression (masked facies), drooling, decreased blinking, and reduced dexterity. Patients with PD may experience difficulty when initiating movement, may freeze or be unable to move when they walk in confined spaces, or may exhibit pill-rolling movements of the hands, jaw, or legs. The tremors and pill-rolling subside on initiation of voluntary movement and do not occur during sleep. Cogwheel-like rigidity, dystonic posture, and postural instability are causes of significant morbidity in patients with PD because they can lead to falls. Patients also exhibit autonomic nervous system dysfunctions such as excessive sweating, constipation, and postural hypotension. Neuropsychologic disorders such as dementia, anxiety, and depression may occur.
Although initially effective, dopaminergic therapies eventually are complicated in most patients by motor fluctuations, including off time (i.e., periods of return of PD symptoms when medication effect wears off) and dyskinesia (i.e., drug-induced involuntary movements such as chorea and dystonia). These motor complications can impair quality of life and cause significant disability. Risk factors for motor complications include younger age at onset of PD, increased disease severity, higher levodopa dosage, and longer disease duration. These problems often are addressed with levodopa adjustments and the addition of adjunctive medications.
Disease staging is an important step in determining drug and supportive therapy. The following scale of Hoehn and Yahr (2001) is the traditional staging tool.
This tool has largely been replaced by the Unified Parkinson Disease Rating Scale (UPDRS) for following the longitudinal course of PD. This latter tool is much more cumbersome but descriptive. It consists of sections on (1) mentation, behavior, and mood; (2) ADLs; and (3) motor activity. These skills are evaluated by interview. Some sections require assignment of multiple grades to each extremity. A total of 199 points is possible, with 199 representing the worst (total) disability and 0 indicating no disability. Both of these tools may be viewed online through the Massachusetts General Hospital neurosurgical website at http://neurosurgery.mgh.harvard.edu/Functional/pdstages.htm.
Mechanism of Action
Drug treatment for PD has centered on increasing the availability of dopamine in the CNS, inhibiting the effects of acetylcholine, and attempting to prevent further cell membrane damage through neuroprotective trials. The D2-receptor subtype is the primary modulator of both clinical improvement and adverse reactions such as dystonia and hallucinations. Increased levodopa precursors and synthesis cofactors usually are not effective.
Levodopa has been the single most important drug in the antiparkinson armamentarium. Levodopa administered orally enters the blood after it is absorbed from the GI tract, and 95% of levodopa is converted to dopamine by L-AAD. The action of this enzyme can be blocked by the antagonist carbidopa, which does not cross the blood-brain barrier; therefore, levodopa in the CNS follows the synthetic path to dopamine formation and storage. Combining carbidopa with levodopa results in increased concentrations of levodopa in the CNS and decreased conversion of L-dopa to dopamine in the periphery, where it causes adverse effects.
Selegiline (also called deprenyl), an MAO-B inhibitor, is used with other drugs early in the management of idiopathic PD. The mechanism of action of selegiline is not fully understood. These drugs inhibit the enzyme MAO-B, which breaks down dopamine in the brain. MAO-B inhibitors cause dopamine to accumulate in surviving nerve cells and reduce the symptoms of PD. Studies supported by the National Institute of Neurological Disorders and Stroke (NINDS) have shown that selegiline can delay the need for levodopa therapy by a year or longer. When selegiline or rasagiline is given with levodopa, they appear to enhance and prolong the response to levodopa and thus may reduce wearing off. Another MAO-B inhibitor, rasagiline, was approved by the FDA in May 2006 for use in treating patients with PD (see Table 46-3). Patients with recent, severe brain injuries treated with daily doses of the Parkinson’s disease drug amantadine hydrochloride improved faster than those in the placebo group, according to a report in The New England Journal of Medicine. “The main finding is that on every single behavioral domain measured, we had a higher incidence of recovery in the amantadine group than in the placebo group,” said Joseph Giacino, who led the research team at JFK Johnson Rehabilitation Institute.
Selective MAO-B inhibition irreversibly blocks the metabolism of dopamine in the brain, where MAO-B is the major subtype and extends the duration of action of L-dopa. Selegiline often permits dose reductions of L-dopa and increases the duration of effect by 1 or more hours in patients who experience “wearing off” effects of L-dopa. Rasagiline may delay the use of carbidopa/levodopa in the early stages of PD via the same mechanism. Rasagiline is classified into two major molecular species, A and B, and is localized in the mitochondrial membranes throughout the body in nerve terminals, brain, liver, and intestinal mucosa. MAO regulates the metabolic degradation of catecholamines and serotonin in the CNS and peripheral tissues. Blockade of MAO-B reduces the metabolism of dopamine, but not that of norepinephrine or serotonin, and inhibits MAO-B in the human brain. The exact mechanism whereby it causes an increase in extracellular levels of dopamine in the striatum is not known.
Dopamine agonists: These drugs, which include bromocriptine, apomorphine, pramipexole, and ropinirole, mimic the role of dopamine in the brain. They can be given alone or in conjunction with levodopa. They may be used in the early stages of the disease, or later on to lengthen the duration of response to levodopa in patients who experience wearing off or on-off effects. They generally are less effective than levodopa in controlling rigidity and bradykinesia.
Dopamine agonists are used in the treatment of patients with IPD to increase the availability of dopamine in the CNS. These drugs work at postsynaptic dopamine receptors in the nigrostriatal system by stimulating dopamine receptors. They are being used more frequently in early parkinsonism to avoid using high doses of levodopa and in late stages of the disease to aid in the management of levodopa dose-response fluctuations.
Pramipexole is a nonergot dopamine agonist with specificity for the D2 dopamine receptors. It binds with lower affinity D3– and D4-receptor subtypes. The relevance of D3-receptor binding in PD is unknown.
Anticholinergic agents are used to control tremors caused by excessive, unopposed acetylcholine. They suppress central cholinergic activity and may inhibit reuptake and storage of dopamine in the CNS, thus prolonging the action of dopamine. They also reduce the incidence and severity of akinesia, rigidity, and tremor by about 20%, and may reduce drooling.
COMT inhibitors are central and/or peripheral blockers of dopamine metabolism. COMT stands for catechol-O-methyltransferase, another enzyme that helps to break down dopamine. Two COMT inhibitors have been approved to treat PD in the United States: entacapone and tolcapone. These drugs, which prolong the effects of levodopa by preventing the breakdown of dopamine, are used as adjuvants with levodopa to extend the therapeutic effect in patients who experience breakthrough tremors prior to their next dose. The mechanism of action of entacapone probably is related to its ability to inhibit COMT and alter the plasma pharmacokinetics of levodopa. The administration of entacapone in conjunction with levodopa and carbidopa produces more sustained plasma levels of levodopa than does the administration of levodopa and carbidopa alone. These sustained plasma levels may result in increased therapeutic effects on the symptoms of PD, as well as increased adverse effects. A decrease in the levodopa dose may be required.
Amantadine has been used for many years and appears to potentiate CNS dopaminergic responses. It may release dopamine and norepinephrine from storage sites and inhibit the reuptake of dopamine and norepinephrine. It is clearly less effective than levodopa but can offer additional benefit in patients experiencing maximal or waning effects from levodopa. It has also been used for the treatment of Sinemet-associated dyskinesia.
Rivastigmine is available as an oral or transdermal system (Exelon Patch) for the treatment of patients with mild to moderate dementia associated with PD. See Chapter 42 for complete prescribing information.
Treatment Principles
Evidence-Based Recommendations
Cardinal Points of Treatment
Nonpharmacologic Treatment
The impact of PD treatment regimens on patients’ health-related quality of life (QOL) is an important health care matter. QOL questionnaires provide insight into the management of disease from the patient’s perspective and help the clinician to assess treatment efficacy. Several studies over the past few years have investigated the QOL of patients with PD; all found depression to be a significant factor in terms of QOL.
Management of PD should include programs designed to optimize the patient’s general health, nutrition, and emotional and neuromuscular status. Table 46-1 provides a treatment algorithm. Refer the patient to a neurologist to confirm the diagnosis and to provide guidance with medical management.
TABLE 46-1
Basic Treatment Algorithm for Parkinson’s Disease
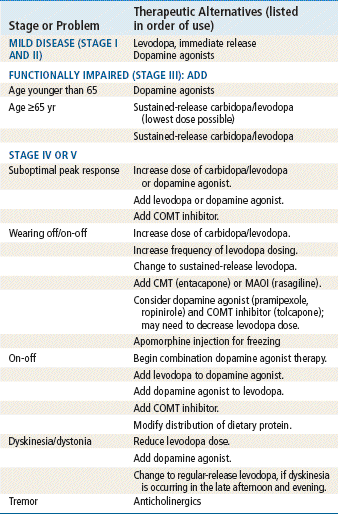
Doses always are titrated slowly according to the patient’s symptoms.
Nonpharmacologic treatment is important. Group support, exercise, education, and nutrition can help. Patients may experience constipation and initially may gain weight if their activity level decreases. With progression of disease, patients may have trouble chewing and swallowing and may need to change their sitting position while eating, taking small bites and chewing well, drinking plenty of water between mouthfuls of food, and avoiding certain foods, such as raw vegetables and nuts, which may be more difficult to chew and swallow. There is some research suggesting that taking caffeine pills equivalent to about three cups of coffee per day may alleviate the motor symptoms in some patients; however, it does not significantly improve daytime sleepiness.
It is customary for levodopa preparations to be dispensed with instructions to take with food. In fact, they may be better and faster absorbed on an empty stomach, but it is often advised that they be taken with food if they upset the stomach. Amino acids in proteins can compete with levodopa (which is also an amino acid) for transport from the gut to the bloodstream, and from the bloodstream into the brain. Most people tolerate levodopa without nausea and are able to take their levodopa medications before meals; for some people, this may occur 1 to 2 hours before eating, whereas others find it better to take their medication 30 minutes before eating.
Some people find that avoiding high-protein foods during the day and “hoarding” them until the evening ensures better mobility during the day. Red meat, poultry, fish, milk, cheese, and eggs all are high in protein. Other individuals benefit from combining small amounts of protein with a high level of carbohydrate (e.g., fruit, bread, cereal, pasta, other grains) throughout the day. Keeping fatty foods to a minimum also may help.
Physical therapy can be very beneficial in keeping the patient mobile and keeping weight down.
Deep brain stimulation has proved effective in controlling symptoms of PD. Other surgical techniques are being used in patients who do not respond to medications or who remain symptomatic.
Pharmacologic Treatment
The NINDS has classified medications for PD into three categories. The first category includes drugs that work directly or indirectly to increase the level of dopamine in the brain. The drugs most commonly used for PD are dopamine precursors—substances such as levodopa that cross the blood-brain barrier and then are changed into dopamine. Other drugs mimic dopamine or prevent or slow its breakdown.
The second category of PD drugs affects other neurotransmitters in the body to ease some symptoms of the disease. For example, anticholinergic drugs interfere with production or uptake of the neurotransmitter acetylcholine. These drugs help to reduce tremors and muscle stiffness, which can result from the presence of more acetylcholine than dopamine.
The third category of drugs prescribed for PD includes medications that help control the nonmotor symptoms of the disease—that is, the symptoms that do not affect movement. For example, people with PD-related depression may be prescribed antidepressants.
The timing of treatment initiation in PD generally is guided by the impact that the disease has on the individual’s quality of life. Over the last decade, a paradigm shift has been noted from initiating symptomatic therapy with levodopa, the gold standard of PD treatment, to beginning treatment with a dopamine agonist (in particular for young-onset patients who are at high risk of developing motor complications) and adding levodopa as a supplement when dopamine agonist monotherapy no longer can provide satisfactory clinical control. Long-term levodopa treatment is associated with the development of motor complications that probably are initiated by abnormal pulsatile stimulation of dopamine receptors via intermittent administration of agents with short half-lives (such as levodopa). Dopamine agonists with longer half-lives can provide relatively continuous stimulation and thus possibly can diminish motor response complications. However, treatment initiation with levodopa still is preferred in patients with PD with cognitive impairment, in the elderly, and in those with atypical parkinsonism. Guidelines put forth by Bhatia et al (2001) also recommend dopamine agonists as alternative first-line treatment options to levodopa for appropriate patients.
The Pawha et al (2006) report, which is a systematic, evidence-based review that was prepared with the goal of improving clinicians’ knowledge of presently available published clinical evidence (based mainly on randomized controlled trials), rated the different therapeutic interventions in terms of efficacy, safety, and clinical usefulness. Safety and efficacy data showed that several drug treatments can be considered as clinically useful or possibly useful for controlling motor features in early and advanced PD. Levodopa was found to be more efficacious than most other medications; orally active dopamine agonists are very similar. The authors also mention the growing consensus on the early use of dopamine agonists, especially in young patients. The choice among available treatment options depends on clinical characteristics such as patient age, disease severity, and the presence of comorbidities, as well as patients’ lifestyle characteristics and preferences, costs of different medications, awareness and perception of available treatment options, and educational background of the treating health care provider.
An immediate-release formulation of levodopa is preferred over a controlled-release preparation for measurement assessment of the therapeutic effect. The switch then can be made to a controlled-release preparation. Therapeutic controversy revolves around the duration of levodopa therapy. Some practitioners believe that levodopa therapy is useful for only approximately 5 years and should be withheld until disabling symptoms appear. The sustained-release formulation of carbidopa/levodopa may provide a more physiologic replacement of dopamine than is provided by other regimens. An immediate-release dose may be added to the morning dose if the patient experiences late onset or an inadequate peak response. The occurrence of blepharospasm or involuntary movement signifies that a dose reduction is needed.
Factors useful in assessing the need to start treatment include which hand is affected (dominant or nondominant), the patient’s employment status, specific Parkinson’s symptoms (bradykinesia is more disabling than tremor), the individual patient’s sentiments, and the individual prescriber’s philosophy. Other factors include the following:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


