Antimicrobial Regimen Selection
KEY CONCEPTS
![]() Every attempt should be made to obtain specimens for culture and sensitivity testing prior to initiating antibiotics.
Every attempt should be made to obtain specimens for culture and sensitivity testing prior to initiating antibiotics.
![]() Empirical antibiotic therapy should be based on knowledge of likely pathogens for the site of infection, information from patient history (e.g., recent hospitalizations, work-related exposure, travel, and pets), and local susceptibility.
Empirical antibiotic therapy should be based on knowledge of likely pathogens for the site of infection, information from patient history (e.g., recent hospitalizations, work-related exposure, travel, and pets), and local susceptibility.
![]() Patients with delayed dermatologic reactions (i.e., rash) to penicillin generally can receive cephalosporins. Patients with type I hypersensitivity reactions (i.e., anaphylaxis) to penicillins should not receive cephalosporins. Alternatives to the cephalosporins include aztreonam, quinolones, sulfonamide antibiotics, or vancomycin based on type of coverage indicated.
Patients with delayed dermatologic reactions (i.e., rash) to penicillin generally can receive cephalosporins. Patients with type I hypersensitivity reactions (i.e., anaphylaxis) to penicillins should not receive cephalosporins. Alternatives to the cephalosporins include aztreonam, quinolones, sulfonamide antibiotics, or vancomycin based on type of coverage indicated.
![]() Creatinine clearance should be estimated for every patient who is to receive antibiotics and the antibiotic dose interval adjusted accordingly. Hepatic function should be considered for drugs eliminated through the hepatobiliary system, such as clindamycin, erythromycin, and metronidazole.
Creatinine clearance should be estimated for every patient who is to receive antibiotics and the antibiotic dose interval adjusted accordingly. Hepatic function should be considered for drugs eliminated through the hepatobiliary system, such as clindamycin, erythromycin, and metronidazole.
![]() All concomitant drugs and nutritional supplements should be reviewed when an antibiotic is added to a patient’s therapy to ensure drug–drug interactions will be avoided.
All concomitant drugs and nutritional supplements should be reviewed when an antibiotic is added to a patient’s therapy to ensure drug–drug interactions will be avoided.
![]() Combination antibiotic therapy may be indicated for polymicrobial infections (e.g., intra-abdominal, gynecologic infections), to produce synergistic killing (such as β-lactam plus aminoglycoside vs. Pseudomonas aeruginosa), or to prevent the emergence of resistance.
Combination antibiotic therapy may be indicated for polymicrobial infections (e.g., intra-abdominal, gynecologic infections), to produce synergistic killing (such as β-lactam plus aminoglycoside vs. Pseudomonas aeruginosa), or to prevent the emergence of resistance.
![]() All patients receiving antibiotics should be monitored for resolution of infectious signs and symptoms (e.g., decreasing temperature and white blood cell count) and adverse drug events.
All patients receiving antibiotics should be monitored for resolution of infectious signs and symptoms (e.g., decreasing temperature and white blood cell count) and adverse drug events.
![]() Antibiotics with the narrowest effective spectrum of activity are preferred. Antibiotic route of administration should be evaluated daily, and conversion from IV to oral therapy should be attempted as signs of infection improve for patients with functioning GI tracts (general exceptions are endocarditis and CNS infections).
Antibiotics with the narrowest effective spectrum of activity are preferred. Antibiotic route of administration should be evaluated daily, and conversion from IV to oral therapy should be attempted as signs of infection improve for patients with functioning GI tracts (general exceptions are endocarditis and CNS infections).
![]() Patients not responding to an appropriate antibiotic treatment in 2 to 3 days should be reevaluated to ensure (a) the correct diagnosis, (b) that therapeutic drug concentrations are being achieved, (c) that the patient is not immunosuppressed, (d) that the patient does not have an isolated infection (i.e., abscess, foreign body), or (e) that resistance has not developed.
Patients not responding to an appropriate antibiotic treatment in 2 to 3 days should be reevaluated to ensure (a) the correct diagnosis, (b) that therapeutic drug concentrations are being achieved, (c) that the patient is not immunosuppressed, (d) that the patient does not have an isolated infection (i.e., abscess, foreign body), or (e) that resistance has not developed.
Choosing an antimicrobial agent to treat an infection is far more complicated than matching a drug to a known or suspected pathogen.1,2 Most clinicians generally follow a systematic approach to select an antimicrobial regimen (Table 83–1). Problems arise when this systematic approach is replaced by prescribing broad-spectrum therapy to cover as many organisms as possible. Consequences of not using the systematic approach include the use of more expensive and potentially more toxic agents, which can, in turn, lead to widespread resistance and difficult-to-treat superinfections. Another abuse of antimicrobial agents is administration when they are not needed such as when they are prescribed for self-limited clinical conditions that are most likely viral in origin (i.e., the common cold).
TABLE 83-1 Systematic Approach for Selection of Antimicrobials
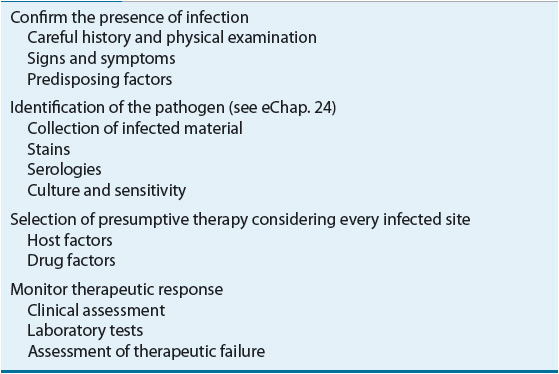
Initial selection of antimicrobial therapy is nearly always empirical, which is prior to documentation and identification of the offending organism. Infectious diseases generally are acute, and a delay in antimicrobial therapy can result in serious morbidity or even mortality. Thus, empirical antimicrobial therapy selection should be based on information gathered from the patient’s history and physical examination and results of Gram stains or of rapidly performed tests on specimens from the infected site. This information, combined with knowledge of the most likely offending organism(s) and an institution’s local susceptibility patterns, should result in a rational selection of antibiotics to treat the patient. This chapter introduces a systematic approach to the selection of antimicrobial therapeutic regimens.
CONFIRMING THE PRESENCE OF INFECTION
Fever
The presence of a temperature greater than the expected 37°C (98.6°F) “normal” body temperature is considered a hallmark of infectious diseases. Body temperature is controlled by the hypothalamus. In addition, the circadian rhythm, a built-in temperature cycle, is also operational. The daily temperature rhythm can vary for each individual. In a healthy person, the internal thermostat is set between the morning low temperature and the afternoon peak as controlled by the circadian rhythm. During fever, the hypothalamus is reset at a higher temperature level.
Fever is defined as a controlled elevation of body temperature above the normal range. The average normal body temperature range taken orally is 36.7°C to 37°C (98°F to 98.6°F). Body temperatures obtained rectally generally are 0.6°C (1°F) higher and axillary temperatures are 0.6°C (1°F) lower than oral temperatures, respectively. Skin temperatures are also less than the oral temperature but can vary depending on the specific measurement method.
Fever can be a manifestation of disease states other than infection. Collagen vascular (autoimmune) disorders and several malignancies can have fever as a manifestation. Fever of unknown or undetermined origin is a diagnostic dilemma and is reviewed extensively elsewhere.3
Many drugs have been identified as causes of fever.4 Drug-induced fever is defined as persistent fever in the absence of infection or other underlying condition. The fever must coincide temporally with the administration of the offending agent and disappear promptly on its withdrawal, after which the temperature remains normal. Possible mechanisms of drug-induced fever are either a hypersensitivity reaction or development of antigen–antibody complexes that result in the stimulation of macrophages and the release of interleukin 1 (IL-1). Although this is not a common drug effect (accounting for no more than 5% of all drug reactions), it should be suspected when obvious reasons for fever are not present. Almost any medication can produce fever, but β-lactam antibiotics, anticonvulsants, allopurinol, hydralazine, nitrofurantoin, sulfonamides, phenothiazines, and methyldopa appear to be responsible more often than others.
Noninfectious etiologies of fever can be referred to as “false-positives.” Although these certainly can confuse the clinician, even more troublesome are false-negatives: the absence of fever in a patient with signs and symptoms consistent with an infectious disease. Careful questioning of the patient or family is vital to assess the ingestion of any medication that can mask fever (e.g., aspirin, acetaminophen, nonsteroidal antiinflammatory agents, and corticosteroids). The use of antipyretics should be discouraged during the treatment of infection unless absolutely necessary because they can mask a poor therapeutic response. Moreover, elevated body temperature, unless very high (>40.5°C [105°F]), is not harmful and may be beneficial.
Signs and Symptoms
White Blood Cell Count
Most infections result in elevated white blood cell (WBC) counts (leukocytosis) because of the increased production and mobilization of granulocytes (neutrophils, basophils, and eosinophils), lymphocytes, or both to ingest and destroy invading microbes. The generally accepted range of normal values for WBC counts is between 4,000 and 10,000 cells/mm3 (4 × 109 and 10 × 109/L). Values above or below this range hold important prognostic and diagnostic value.
Bacterial infections are associated with elevated granulocyte counts, often with immature forms (band neutrophils) seen in peripheral blood smears. Mature neutrophils are also referred to as segmented neutrophils or polymorphonuclear (PMN) leukocytes. The presence of immature forms (left shift) is an indication of an increased bone marrow response to the infection. With infection, peripheral WBC counts can be very high, but they are rarely higher than 30,000 to 40,000 cells/mm3 (30 × 109 to 40 × 109/L). Because leukocytosis indicates the normal host response to infection, low leukocyte counts after the onset of infection indicate an abnormal response and generally are associated with a poor prognosis.
The most common granulocyte defect is neutropenia, a decrease in absolute numbers of circulating neutrophils. A thorough description of the consequences of neutropenia is given in Chapter 99. Lymphocytosis, even with normal or slightly elevated total WBC counts, generally is associated with tuberculosis and viral or fungal infections. Increases in monocytes can be associated with tuberculosis or lymphoma, and increases in eosinophils can be associated with allergic reactions to drugs or infections caused by metazoa. Many types of infections can be accompanied by a completely normal WBC count and differential.
Local Signs
The classic signs of pain and inflammation can manifest as swelling, erythema, tenderness, and purulent drainage. Unfortunately, these are only visible if the infection is superficial or in a bone or joint. The manifestations of inflammation in deep-seated infections (e.g., meningitis, pneumonia, endocarditis, and urinary tract infection) must be ascertained by examining tissues or fluids. For example, the presence of neutrophils in spinal fluid, lung secretions (sputum), or urine is highly suggestive of a bacterial infection.
Symptoms referable to an organ system must be sought out carefully because not only do they help in establishing the presence of infection, but they also aid in narrowing the list of potential pathogens. For example, a febrile patient with complaints of flank pain and dysuria can well have pyelonephritis. In this situation, enteric gram-negative bacilli, especially Escherichia coli, are the predominant pathogens. If a febrile patient has no symptoms suggestive of an organ system but only constitutional complaints, the list of possible infectious diseases is lengthy.3 A febrile individual with cough and sputum production probably has a pulmonary infection. What is not so evident, however, is the etiologic organism in this situation, because it can be caused by bacteria, mycobacteria, viruses, Chlamydia, or mycoplasmas.5 In this situation, attention to the patient’s history and background disease states is important. Even more important is a careful examination of the infected material (in this case sputum) to ascertain the identity of the pathogen.
IDENTIFICATION OF THE PATHOGEN
Microbiology Issues
![]() Infected body materials must be sampled, if at all possible or practical, before institution of any antimicrobial therapy for two reasons. First, a Gram stain of the material might reveal bacteria, or an acid-fast stain might detect mycobacteria or actinomycetes. Second, a delay in obtaining infected fluids or tissues until after antimicrobial therapy is started might result in false-negative culture results or alterations in the cellular and chemical composition of infected fluids. This is particularly true in patients with urinary tract infections, meningitis, and septic arthritis.6
Infected body materials must be sampled, if at all possible or practical, before institution of any antimicrobial therapy for two reasons. First, a Gram stain of the material might reveal bacteria, or an acid-fast stain might detect mycobacteria or actinomycetes. Second, a delay in obtaining infected fluids or tissues until after antimicrobial therapy is started might result in false-negative culture results or alterations in the cellular and chemical composition of infected fluids. This is particularly true in patients with urinary tract infections, meningitis, and septic arthritis.6
Blood cultures usually should be performed in the acutely ill febrile patient. Blood culture collection should coincide with sharp elevations in temperature, suggesting the possibility of microorganisms or microbial antigens in the bloodstream. Ideally, blood should be obtained from peripheral sites as two sets (one set consists of an aerobic bottle and one set an anaerobic bottle) from two different sites approximately 1 hour apart. In selected infections, bacteremia is qualitatively continuous (e.g., endocarditis), so cultures can be obtained at any time.7
In addition to the infected materials produced by the patient (e.g., blood, sputum, urine, stool, and wound or sinus drainage), other less accessible fluids or tissues must be obtained if they are suspected to be the infected site (e.g., spinal fluid in meningitis and joint fluid in arthritis). Abscesses and cellulitic areas also should be aspirated.
Interpreting Results
After a positive Gram stain, culture results, or both are obtained, the clinician must be cautious in determining whether the organism recovered is a true pathogen, a contaminant, or a part of the normal flora (see eChap. 24). The latter consideration is especially problematic with cultures obtained from the skin, oropharynx, nose, ears, eyes, throat, and perineum. These surfaces are heavily colonized with a wide variety of bacteria, some of which can be pathogenic in certain settings. For example, coagulase-negative staphylococci are found in cultures of all the aforementioned sites, yet are seldom regarded as pathogens unless recovered from blood, venous access catheters, or prosthetic devices.
Importantly, cultures of specimens from purportedly infected sites that are obtained by sampling from or through one of these contaminated areas might contain significant numbers of the normal flora. For urine cultures, the urinalysis should be used in combination with culture results to assess the presence of WBCs, nitrite, and leukocyte esterase to help confirm infection and rule out colonization.
Particularly problematic are expectorated sputum specimens that must be evaluated carefully by determination of the presence of squamous epithelial cells and leukocytes.5 A predominance of epithelial cells in sputum specimens reduces the likelihood that recovered bacteria are pathogenic, especially when multiple types of organisms are seen on Gram stain. In contrast, the discovery of leukocytes in large numbers with one predominant type of organism is a more reliable indicator of a valid collection. In general, however, sputum evaluation has poor sensitivity and specificity as a diagnostic test.5
Caution also must be used in the evaluation of positive culture results from normally sterile sites (e.g., blood, cerebrospinal fluid [CSF], or joint fluid). The recovery of bacteria normally found on the skin in large quantities (e.g., coagulase-negative staphylococci or diphtheroids) from one of these sites can be a result of contamination of the specimen rather than a true infection. However, these organisms can be pathogenic in certain settings.
Gram-staining techniques, culture methods, and serologic identification, as well as susceptibility testing, are discussed in detail in eChapter 24. Emphasis must be placed on the proper collection and handling of specimens and careful assessment of Gram stain or other test results in guiding the clinician toward appropriate selection of initial antimicrobial therapy.8
SELECTION OF PRESUMPTIVE THERAPY
![]() To select rational antimicrobial therapy for a given clinical situation, a variety of factors must be considered. These include the severity and acuity of the disease, host factors, factors related to the drugs used, and the necessity for using multiple agents. In addition, there are generally accepted drugs of choice for the treatment of most pathogens (see Appendix 83–1).
To select rational antimicrobial therapy for a given clinical situation, a variety of factors must be considered. These include the severity and acuity of the disease, host factors, factors related to the drugs used, and the necessity for using multiple agents. In addition, there are generally accepted drugs of choice for the treatment of most pathogens (see Appendix 83–1).
Drugs of choice are compiled from a variety of sources and are intended as guidelines rather than as specific rules for antimicrobial use. These choices are influenced by local antimicrobial susceptibility data rather than information published by other institutions or national compilations. Each institution should publish an annual summary of antibiotic susceptibilities (antibiogram) for organisms cultured from patients. Antibiograms contain both the number of nonduplicate isolates for common species and the percentage susceptible to the antibiotics tested. To further guide empirical antibiotic therapy, some hospitals publish unit-specific antibiograms in unique patient care areas, such as intensive care units or burn units.
Susceptibility of bacteria can differ substantially among hospitals within a community. For example, the prevalence of hospital-acquired methicillin-resistant Staphylococcus aureus (HA-MRSA) in some centers is quite high, whereas in other centers the problem might be nonexistent. This particular situation will influence the selection of therapy for possible S. aureus infection, where the clinician must choose either a β-lactam or vancomycin. The problem of differing susceptibilities is not limited only to gram-positive bacteria but also is evident in gram-negative organisms, and all drug classes are affected.
Empirical therapy is directed at organisms that are known to cause the infection in question. These organisms are discussed for different sites of infection in Chapters 83 to 102. To define the most likely infecting organisms, a careful history and physical examination must be performed. The place where the infection was acquired should be determined, for example, the home (community acquired), nursing home environment, or hospital acquired (nosocomial). Nursing home patients can be exposed to potentially more resistant organisms because they are often surrounded by ill patients who are receiving antibiotics. Other important questions to ask infected patients regarding the history of present illness include the following:
1. Are any other people sick at home, especially children?
2. Are any unusual pets kept in the home such as pigeons?
3. Where are you employed (i.e., are you exposed to contaminated meat or infectious biohazards)?
4. Has there been any recent travel (i.e., to endemic areas of fungal infections or developing countries)?
Host Factors
Several host factors should be considered when evaluating a patient for antimicrobial therapy. The most important factors are drug allergies, age, pregnancy, genetic or metabolic abnormalities, renal and hepatic function, site of infection, concomitant drug therapy, and underlying disease states.
Allergy
![]() Allergy to an antimicrobial agent generally precludes its use. Careful assessment of allergy histories must be performed because many patients confuse common adverse drug effects (i.e., GI disturbance) with true allergic reactions.9 Among the most commonly cited antimicrobial allergies are those to penicillin, penicillin-related compounds, or both. In the absence of complete penicillin skin testing capabilities, a rule of thumb for giving cephalosporins to patients allergic to penicillin is to avoid giving them to patients who give a good history for immediate or accelerated reactions (e.g., anaphylaxis, laryngospasm) and to give them under close supervision in patients with a history of delayed reactions, such as a rash.10 If a gram-negative infection is suspected or documented, therapy with a monobactam may be appropriate because cross-reactivity with other β-lactams is nonexistent.
Allergy to an antimicrobial agent generally precludes its use. Careful assessment of allergy histories must be performed because many patients confuse common adverse drug effects (i.e., GI disturbance) with true allergic reactions.9 Among the most commonly cited antimicrobial allergies are those to penicillin, penicillin-related compounds, or both. In the absence of complete penicillin skin testing capabilities, a rule of thumb for giving cephalosporins to patients allergic to penicillin is to avoid giving them to patients who give a good history for immediate or accelerated reactions (e.g., anaphylaxis, laryngospasm) and to give them under close supervision in patients with a history of delayed reactions, such as a rash.10 If a gram-negative infection is suspected or documented, therapy with a monobactam may be appropriate because cross-reactivity with other β-lactams is nonexistent.
Age
The patient’s age is an important factor both in trying to identify the likely etiologic agent and in assessing the patient’s ability to eliminate the drug(s) to be used. The best example of an age determinant of organisms is in bacterial meningitis, where the pathogens differ as the patient grows from the neonatal period through infancy and childhood into adulthood.6
For neonates, hepatic and liver functions are not well developed. Therefore, bilirubin excretion is decreased resulting in increased concentration of unconjugated bilirubin that can cause kernicterus. Neonates (especially when premature) can develop kernicterus when given sulfonamides. This results from displacement of bilirubin from serum albumin. In addition, neonates have more body water content that results in a larger volume of distribution leading to adjustments in antibiotic dosing regimens. Additional special drug considerations for pediatric patients include low frequency of adverse effects and compliance-enhancing features (e.g., absorption not affected by food, once- to twice-daily dosing, and good taste).11
The major physiologic change in persons older than 65 years of age is a decline in the number of functioning nephrons that, in turn, results in decreased renal function.12 This is usually manifested by an increased incidence of side effects caused by antimicrobials that are eliminated renally. For example, renal toxicity caused by aminoglycosides may be apparent much sooner during therapy than in younger patients.
Pregnancy
During pregnancy, not only is the fetus at risk for drug teratogenicity, but also the pharmacokinetic disposition of certain drugs can be altered.13 Penicillins, cephalosporins, and aminoglycosides are cleared from the peripheral circulation more rapidly during pregnancy. This is probably a result of marked increases in intravascular volume, glomerular filtration rate, and hepatic and metabolic activities. The net result is that maternal serum antimicrobial concentrations can be as much as 50% lower during this period than in the nonpregnant state. Increased dosages of certain compounds might be necessary to achieve therapeutic levels during late pregnancy.
Metabolic or Genetic Variation
Inherited or acquired metabolic abnormalities will influence the therapy of infectious diseases in a variety of ways. For example, patients with impaired peripheral vascular flow may not absorb drugs given by intramuscular injection. In addition, certain metabolic states can predispose patients to enhanced drug toxicity. For instance, patients who are phenotypically slow acetylators of isoniazid are at greater risk for peripheral neuropathy.14 Patients with severe deficiency of glucose-6-phosphate dehydrogenase can develop significant hemolysis when exposed to such drugs as sulfonamides, nitrofurantoin, nalidixic acid, antimalarials, and dapsone. Although mild deficiencies are found in African Americans, the more severe forms of the disease generally are confined to persons of eastern Mediterranean origin. Another example is the antiretroviral drug abacavir, which is associated with a severe hypersensitivity reaction, consisting of fever, rash, abdominal pain, and respiratory distress. This risk has been associated with the presence of a human leukocyte antigen allele HLA-B*5701. Routine screening for the presence of this allele before initiating treatment with abacavir is a recommendation in the current HIV treatment guidelines.
Organ Dysfunction
![]() Patients with diminished renal or hepatic function or both will accumulate certain drugs unless the dosage is adjusted.15,16 Recommendations for dosing antibiotics in patients with liver dysfunction are not as formalized as guidelines for patients with renal dysfunction. Antibiotics that should be adjusted in severe liver disease include clindamycin, erythromycin, metronidazole, and rifampin. Significant accumulation can occur when both liver dysfunction and renal dysfunction are present for the following drugs: cefotaxime, nafcillin, piperacillin, and sulfamethoxazole.
Patients with diminished renal or hepatic function or both will accumulate certain drugs unless the dosage is adjusted.15,16 Recommendations for dosing antibiotics in patients with liver dysfunction are not as formalized as guidelines for patients with renal dysfunction. Antibiotics that should be adjusted in severe liver disease include clindamycin, erythromycin, metronidazole, and rifampin. Significant accumulation can occur when both liver dysfunction and renal dysfunction are present for the following drugs: cefotaxime, nafcillin, piperacillin, and sulfamethoxazole.
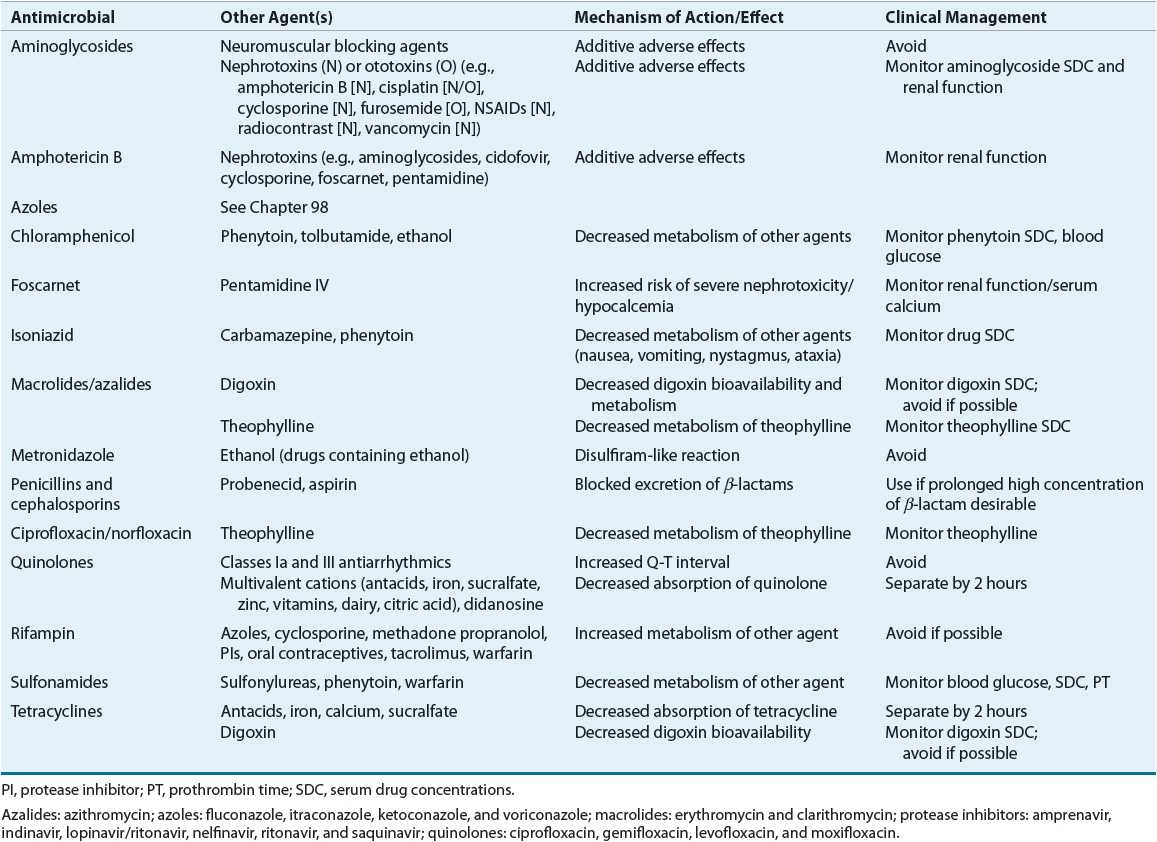
Concomitant Drugs
![]() Any concomitant therapy that the patient is receiving can influence the drug selection, dose, and monitoring. For instance, administration of isoniazid to a patient who is also receiving phenytoin can result in phenytoin toxicity secondary to inhibition of phenytoin metabolism by isoniazid. Furthermore, drugs that possess similar adverse effect profiles can increase the risk for effects (i.e., two drugs that cause nephrotoxicity or neutropenia). A detailed review of drug interactions is beyond the scope of this chapter, but an excellent textbook on this subject is available.17 Lists of potentially severe drug–drug interactions are provided in Table 83–2.
Any concomitant therapy that the patient is receiving can influence the drug selection, dose, and monitoring. For instance, administration of isoniazid to a patient who is also receiving phenytoin can result in phenytoin toxicity secondary to inhibition of phenytoin metabolism by isoniazid. Furthermore, drugs that possess similar adverse effect profiles can increase the risk for effects (i.e., two drugs that cause nephrotoxicity or neutropenia). A detailed review of drug interactions is beyond the scope of this chapter, but an excellent textbook on this subject is available.17 Lists of potentially severe drug–drug interactions are provided in Table 83–2.
TABLE 83-2 Major Drug Interactions with Antimicrobials