KEY CONCEPTS
![]() Prophylactic antibiotic therapy differs from presumptive and therapeutic antibiotic therapy in that the latter two involve treatment regimens for documented or presumed infections, whereas the goal of prophylactic therapy is to prevent infections in high-risk patients or procedures.
Prophylactic antibiotic therapy differs from presumptive and therapeutic antibiotic therapy in that the latter two involve treatment regimens for documented or presumed infections, whereas the goal of prophylactic therapy is to prevent infections in high-risk patients or procedures.
![]() The risk of a surgical site infection (SSI) is determined from both the type of surgery and the patient-specific risk factors; however, most commonly used classification systems account for only procedure-related risk factors.
The risk of a surgical site infection (SSI) is determined from both the type of surgery and the patient-specific risk factors; however, most commonly used classification systems account for only procedure-related risk factors.
![]() The timing of antimicrobial prophylaxis is of paramount importance. Antibiotics should be administered within 1 hour before surgery to ensure adequate drug levels at the surgical site prior to the initial incision.
The timing of antimicrobial prophylaxis is of paramount importance. Antibiotics should be administered within 1 hour before surgery to ensure adequate drug levels at the surgical site prior to the initial incision.
![]() Antimicrobial agents with short half-lives (e.g., cefazolin) may require intraoperative redosing during long (>3 hours) procedures.
Antimicrobial agents with short half-lives (e.g., cefazolin) may require intraoperative redosing during long (>3 hours) procedures.
![]() The type of surgery, intrinsic patient risk factors, most commonly identified pathogenic organisms, institutional antimicrobial resistance patterns, and cost must be considered when choosing an antimicrobial agent for prophylaxis.
The type of surgery, intrinsic patient risk factors, most commonly identified pathogenic organisms, institutional antimicrobial resistance patterns, and cost must be considered when choosing an antimicrobial agent for prophylaxis.
![]() Single-dose prophylaxis is appropriate for many types of surgery. First-generation cephalosporins (e.g., cefazolin) are the mainstay for prophylaxis in most surgical procedures because of their spectrum of activity, safety, and cost.
Single-dose prophylaxis is appropriate for many types of surgery. First-generation cephalosporins (e.g., cefazolin) are the mainstay for prophylaxis in most surgical procedures because of their spectrum of activity, safety, and cost.
![]() Vancomycin as a prophylactic agent should be limited to patients with a documented history of life-threatening β-lactam hypersensitivity or those in whom the incidence of infections with organisms resistant to cefazolin (e.g., methicillin-resistant Staphylococcus aureus) is documented or high enough to justify use.
Vancomycin as a prophylactic agent should be limited to patients with a documented history of life-threatening β-lactam hypersensitivity or those in whom the incidence of infections with organisms resistant to cefazolin (e.g., methicillin-resistant Staphylococcus aureus) is documented or high enough to justify use.
According to the National Center for Health Statistics, some 46 million surgical procedures are performed annually in the United States, the majority of which are done in an outpatient setting.1 Infection is the most common complication of surgery.2 Surgical site infections (SSIs) occur in – 3% to 6% of patients and prolong hospitalization by an average of 7 days at a direct annual cost of $5 billion to $10 billion.3,4 SSIs are the third (14% to 16%) most frequent cause of nosocomial infections among hospitalized patients and the primary (40%) cause of nosocomial infection in surgical patients.3 Prophylactic administration of antibiotics decreases the risk of infection after many surgical procedures and represents an important component of care for this population.
Antibiotics administered prior to the contamination of previously sterile tissues or fluids are called prophylactic antibiotics. The goal of therapy is to prevent an infection from developing. Although eradication of distal (preexisting, unrelated to surgery) infections lowers the risk for subsequent postoperative infections, it does not per se constitute a prophylactic regimen. In fact, surgical prophylaxis often is prescribed concurrently under these circumstances because of important antimicrobial spectrum- and timing-related concerns. Both SSIs and infections not directly related to the surgical site (e.g., urinary tract infections and pneumonia) are termed nosocomial. Prevention of hospital-acquired infections is a major goal of antibiotic prophylaxis.
![]() Presumptive antibiotic therapy is administered when an infection is suspected but not yet proven. Clinical scenarios where presumptive therapy is used commonly include acute cholecystitis, open compound fractures, and acute appendicitis of less than 24 hours’ duration. In these situations, if signs of perforation or infection are absent during surgery, then routine prophylactic treatment rather than presumptive therapy is warranted. An operative finding of a gangrenous gallbladder or a perforated appendix, however, is suggestive of an established infectious process, and a therapeutic antibiotic regimen is required.3
Presumptive antibiotic therapy is administered when an infection is suspected but not yet proven. Clinical scenarios where presumptive therapy is used commonly include acute cholecystitis, open compound fractures, and acute appendicitis of less than 24 hours’ duration. In these situations, if signs of perforation or infection are absent during surgery, then routine prophylactic treatment rather than presumptive therapy is warranted. An operative finding of a gangrenous gallbladder or a perforated appendix, however, is suggestive of an established infectious process, and a therapeutic antibiotic regimen is required.3
According to the Centers for Disease Control and Prevention’s (CDC) National Nosocomial Infections Surveillance System (NNIS),3 SSIs can be categorized as either incisional (e.g., cellulitis of the incision site) or organ/space (e.g., meningitis; Fig. 101-1). Incisional SSIs are subcategorized into superficial (involving only the skin or subcutaneous tissue) and deep (fascial and muscle layers) infections. Organ/space SSIs can involve any anatomic area other than the incision site. For example, a patient who develops bacterial peritonitis after bowel surgery has an organ/space SSI. By definition, SSIs must occur within 30 days of surgery. If a prosthetic implant is involved, a deep incisional or organ/space SSI can be reported up to 1 year from the date of surgery. Although microbiologic testing of surgical drainage material or sites may help to guide care, the specificity of a negative culture is poor and generally does not rule out an SSI.3
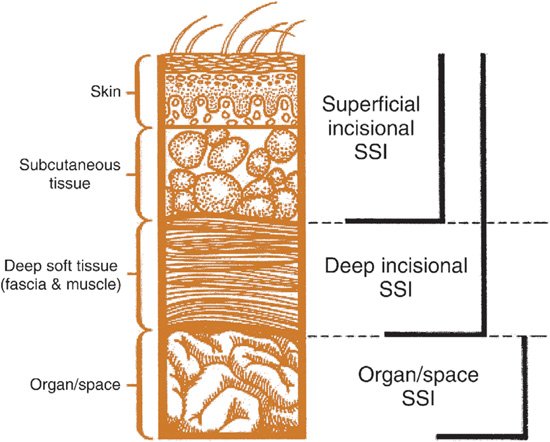
FIGURE 101-1 Cross section of abdominal wall depicting Centers for Disease Control and Prevention classifications of surgical site infections (SSI). (Reprinted from reference 3 with permission from Elsevier and the Association for Professionals in Infection Control and Epidemiology.)
RISK FACTORS FOR SURGICAL SITE INFECTIONS
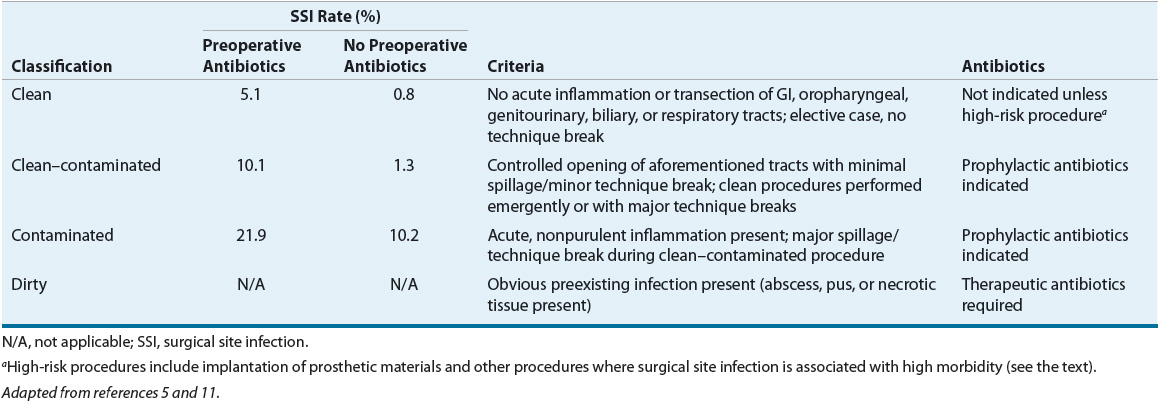
![]() SSI incidence depends on both procedure- and patient-related factors. Traditionally, the risk for SSIs has been stratified by surgical procedure in a classification system developed by the National Research Council (NRC; Table 101-1).5 The NRC classification system proposes that the risk of an SSI depends on the microbiology of the surgical site, the presence of a preexisting infection, the likelihood of contaminating previously sterile tissue during surgery, and the events during and after surgery.5,6 A patient’s NRC procedure classification is the primary determinant of whether antibiotic prophylaxis is warranted. However, because a patient’s NRC wound classification is influenced by surgical findings (e.g., gangrenous gallbladder) and perioperative events (e.g., major technique breaks), categorization generally occurs intraoperatively.7
SSI incidence depends on both procedure- and patient-related factors. Traditionally, the risk for SSIs has been stratified by surgical procedure in a classification system developed by the National Research Council (NRC; Table 101-1).5 The NRC classification system proposes that the risk of an SSI depends on the microbiology of the surgical site, the presence of a preexisting infection, the likelihood of contaminating previously sterile tissue during surgery, and the events during and after surgery.5,6 A patient’s NRC procedure classification is the primary determinant of whether antibiotic prophylaxis is warranted. However, because a patient’s NRC wound classification is influenced by surgical findings (e.g., gangrenous gallbladder) and perioperative events (e.g., major technique breaks), categorization generally occurs intraoperatively.7
TABLE 101-1 National Research Council Wound Classification, Risk of Surgical Site Infection, and Indication for Antibiotics
Inherent Patient Risk
The NRC classification system does not account for the influence of underlying patient risk factors for SSI development, instead categorizing the risks for SSIs simply based on a specific surgical procedure. Disease states and conditions known to increase SSI risk are listed in Table 101-2. Preexisting distal infections increase SSI rates and should be resolved prior to surgery whenever possible. Diabetic patients have an increased risk for SSIs, especially those with uncontrolled perioperative blood sugars. Preoperative smoking has been identified as an independent risk factor for SSI because of the deleterious effects of nicotine on wound healing. Preoperative immunosuppression, including corticosteroid use, may increase infection risk. Patients coinfected with human immunodeficiency virus (HIV) and hepatitis C are at approximately double the risk of SSI as the general population.8 Malnutrition is a well-described risk factor for postoperative complications, including SSI, impaired wound and colonic anastomosis healing, and prolonged hospital stay. Although enteral feeding during the perioperative period can reduce bacterial translocation by maintaining the integrity of the intestinal mucosa, nutritional supplementation does not decrease the incidence of infection.9
TABLE 101-2 Patient and Operation Characteristics That May Influence the Risk of Surgical Site Infection
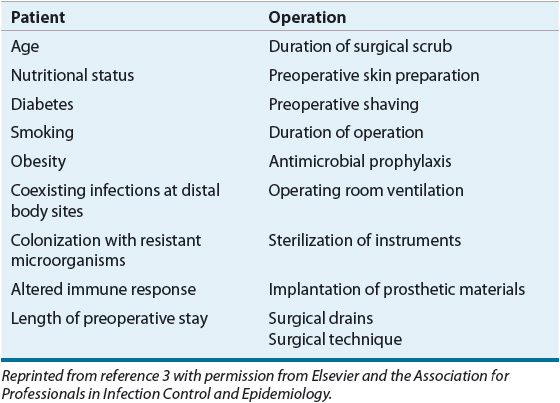
Colonization of the nares with Staphylococcus aureus is a well-described SSI risk factor.3 Although intranasal application of mupirocin ointment reduces the rate of nasal carriage of S. aureus, one large, randomized, double-blind study of 4,030 surgical patients found that prophylactic intranasal mupirocin did not reduce the rate of S. aureus SSI, although it did reduce the rate of nosocomial S. aureus infections among patients who were S. aureus carriers.10 Other factors shown to increase the risk of SSI are age, length of preoperative hospital stay, and obesity.3
Identifying SSI Risk
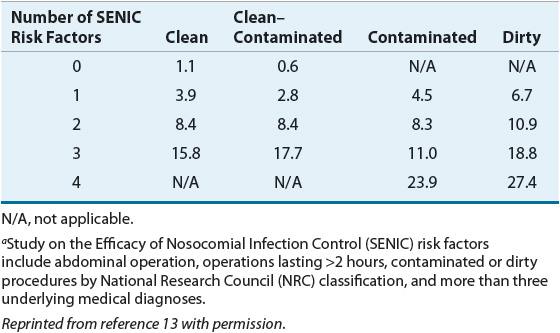
Two large epidemiologic studies have objectively quantified SSI risk based on specific patient- and procedure-related factors. The Study on the Efficacy of Nosocomial Infection Control (SENIC) analyzed more than 100,000 surgery cases to identify and validate risk factors for SSI.11 Abdominal operations, operations lasting longer than 2 hours, contaminated or “dirty” procedures (as per NRC classification), and more than three underlying medical diagnoses each was associated with an increased incidence of SSI. When NRC classification was stratified by number of SENIC risk factors present, SSI incidence varied by as much as a factor of 15 within the same NRC operative category (Table 101-3).12
TABLE 101-3 Surgical Site Infection Incidence (%) Stratified by NRC Wound Classification and SENIC Risk Factorsa
In a subsequent analysis of more than 84,000 surgical cases, the NNIS attempted to simplify and refine the SENIC system by quantifying intrinsic patient risk using the American Society of Anesthesiologists’ (ASA) preoperative assessment score (Table 101-4).14,15 An ASA score ≥3 was a strong predictor for the development of an SSI. Other factors associated with increased SSI incidence are contaminated or “dirty” operations (NRC criteria) and surgical procedures lasting longer than average. As in the SENIC study, the SSI rate was linked to the number of risk factors present and varied considerably within NRC class. The NNIS basic SSI risk index is composed of the following criteria: ASA score = 3, 4, or 5; wound class; and duration of surgery. Overall, for 34 of the 44 NNIS procedure categories, SSI rates increased proportionally with the number of risk factors present.13 The SSI rate was generally lower when the procedure was done laparoscopically.
TABLE 101-4 American Society of Anesthesiologists’ Physical Status Classification

Although evidence-based recommendations for antimicrobial prophylaxis during surgery are best established using the results of randomized clinical trials, many studies have small sample sizes and do not stratify patients according to overall SSI risk. Future studies, particularly those involving clean procedures, should be stratified by SSI risk so that the subset of high-risk patients who might benefit the most from prophylaxis is clearly established.
BACTERIOLOGY
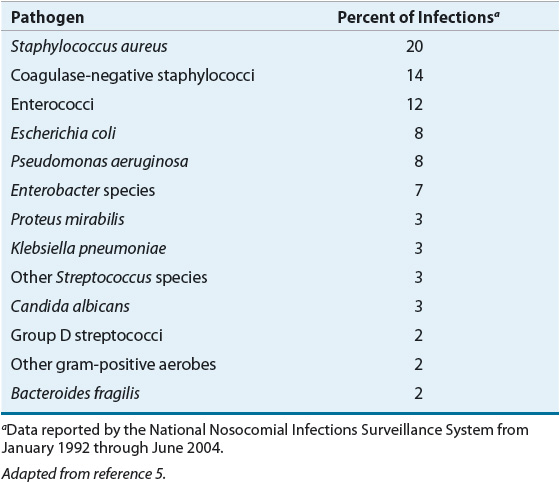
The most important consideration when choosing antibiotic prophylaxis is the bacteriology of the surgical site. Organisms involved in an SSI are acquired by one of two ways: endogenously (from the patient’s own normal flora) or exogenously (from contamination during the surgical procedure). Based on the type and anatomic location of the procedure and the NRC classification (see Table 101-1), resident flora can be predicted and appropriate antibiotic choices made. According to NNIS data, S. aureus, coagulase-negative staphylococci, enterococci, Escherichia coli, and Pseudomonas aeruginosa are the pathogens most commonly isolated (Table 101-5).14 With the widespread use of broad-spectrum antibiotics, however, Candida species and methicillin-resistant Staphylococcus aureus (MRSA) are becoming more prevalent.14
TABLE 101-5 Major Pathogens in Surgical Wound Infections
Factors affecting the ability of an organism to induce an SSI depend on organism count, organism virulence, and host immunocompetency. Organisms in the commensal flora generally are not pathogenic. These organisms often serve the host as a form of protection against invasive organisms that otherwise would colonize the surgical site. Opportunistic organisms usually are kept in check by normal flora and rarely are problematic unless they are present in large numbers. The loss of normal flora through the use of broad-spectrum antibiotics can destabilize homeostasis, allowing pathogenic bacteria to proliferate and infection to occur.4
Normal flora translocated to a normally sterile tissue site or fluid during a surgical procedure can become pathogenic. For example, S. aureus or Staphylococcus epidermidis may be translocated from the surface of the skin to deeper tissues or E. coli from the colon to the peritoneal cavity, bloodstream, or urinary tract. Studies in animals and healthy volunteers have shown bacterial virulence to be an important determinant in the development of secondary infections.16,17 Whereas more than one million S. aureus per square centimeter or gram of tissue are required to produce infection in animals, less than 100,000 Streptococcus pyogenes per square centimeter or gram of tissue are required at the same site.17,18
Impaired host defense reduces the number of bacteria required to establish an infection. A breach of normal host defenses through surgical intervention (e.g., insertion of a prosthetic device) may enable organisms to cause infection. In addition, the loss of specific immune factors, such as complement activation, tissue-derived inhibitors (e.g., proinflammatory cytokines), cell-mediated response (e.g., T-cell function), and granulocytic or phagocytic function (e.g., neutrophils or macrophages) can greatly increase the risk for SSI development.19 Vascular occlusive states related to the surgical procedure or those occurring from hypovolemic shock can greatly affect blood flow to the surgical site, thus diminishing host defense mechanisms against microbial invasion. Traumatized tissue, hematomas, and the presence of foreign material also lead to more infections. When a foreign body is introduced during a surgical procedure, fewer than 100 bacterial colony-forming units are required to cause an SSI.20 Studies examining S. aureus-contaminated wound infections on the skin of healthy volunteers demonstrate a 10,000-fold reduction in the number of organisms required to establish a wound infection if sutures are not present.16
ANTIMICROBIAL RESISTANCE
Colonization of the host with antibiotic-resistant hospital flora prior to or during surgery may lead to an SSI that is unresponsive to routine antibiotic therapy. The most common cause of nosocomially acquired multiresistant organisms is transmission from hospital personnel.21 Patients treated with broad-spectrum antibiotic therapy are at increased risk for colonization with hospital flora.
With cephalosporins established as first-line agents for prophylaxis, organisms resistant to cephalosporins represent the majority of pathogens causing SSIs. MRSA and coagulase-negative staphylococci have emerged as the most common pathogens in patients who develop SSIs despite prophylaxis with cephalosporins particularly in cardiothoracic, vascular, orthopedic, and neurologic surgery. Methicillin resistance not only limits the treatment/prophylaxis options available, but it also is associated with increased mortality, longer hospital lengths of stay, and increased costs.22,23 Although the use of vancomycin for prophylaxis may be appropriate for some operations performed in hospitals with a high rate of infection due to MRSA, there is little guidance on what constitutes a “high rate” of MRSA infection and whether providing prophylaxis with vancomycin alone will result in fewer SSIs.24 A more effective strategy would be to screen elective surgical candidates for MRSA colonization preoperatively. MRSA colonization is predictive of MRSA SSI and thus effective prophylaxis with vancomycin is then reserved for carriers only. Some single center studies evaluating the decolonization of MRSA carriers preoperatively (i.e., with intranasal mupirocin, chlorhexidine showers) yield mixed results and may not be cost-effective.25,26
Although cefazolin remains a mainstay in cardiovascular SSI prophylaxis, its failure has been reported in cases involving methicillin-sensitive Staphylococcus aureus (MSSA). In a comparison trial between cefamandole and cefazolin, significantly more failures were attributed to cefazolin, even though the primary pathogen was MSSA.27 However, a similar trial comparing cefazolin and cefuroxime did not show any difference in SSI incidence between the two regimens.27 The β-lactamase expressed by some MSSA may be capable of hydrolyzing cefazolin more readily than cefuroxime or cefamandole. Although this trend is disturbing, the overall incidence of cefazolin failure remains low, and cefazolin remains the drug of choice for SSI prophylaxis in cardiovascular surgery.27
The increase in frequency of fungal infections in surgical patients has drawn concern. In hospitalized patients, the incidence of nosocomial Candida infections nearly doubled from 1992 to 2004.14,28 Overzealous use of broad-spectrum antibiotics is the most likely cause for this increase. A study of patients undergoing cardiovascular surgery identified sex (female), length of stay in the ICU, and duration of central venous catheterization as risk factors for postoperative Candida infections.29 Although presurgical Candida colonization is associated with a higher risk of fungal SSIs, routine preoperative use of prophylactic antifungal agents is not being advocated at this time.28,30
SCHEDULING ANTIBIOTIC ADMINISTRATION
![]()
![]() The following principles must be considered when providing antimicrobial surgical prophylaxis: (a) the agents should be delivered to the surgical site prior to the initial incision, and (b) bactericidal antibiotic concentrations should be maintained at the surgical site throughout the surgical procedure. Although animal and human models have demonstrated the efficacy of a single dose of an antibiotic administered just prior to bacterial contamination, long operations often require intraoperative doses of antibiotics to maintain adequate concentrations at the surgical site for the duration of surgery.31 Antibiotics should be administered with anesthesia just prior to the initial incision. Administration of antibiotics too early may result in concentrations below the MIC toward the end of the operation, and administration too late leaves the patient unprotected at the time of initial incision. In a study examining the timing of antibiotic administration to 2,847 patients receiving prophylaxis, Classen et al.31 evaluated patients who received prophylaxis early (2 to 24 hours before surgery), preoperative prophylaxis (0 to 2 hours prior to surgery), perioperative prophylaxis (up to 3 hours after first incision), and postoperative prophylaxis (>3 hours after the first incision). The risk of infection was lowest (0.6%) for patients who received preoperative prophylaxis, moderate (1.4%) for those who received perioperative antibiotics, and greatest for those who received postoperative antibiotics (3.3%) or preoperative antibiotics too early (3.8%). The risk for an SSI increases dramatically with each hour from the time of initial incision to the time when antibiotics are eventually administered. For these reasons, prophylactic antibiotics should not be prescribed to be given “on call to the operating room (OR),” which can occur two or more hours prior to the initial incision, nor should concurrent therapeutic antibiotics be relied on to provide adequate protection. In both situations, the chance for improperly timed doses is high. Although the landmark study by Classen et al.31 confirmed that antimicrobial prophylaxis should be administered within 2 hours prior to the initial incision, administration immediately prior to the incision may not allow enough time for the drug to distribute throughout the tissues involved in the surgery.
The following principles must be considered when providing antimicrobial surgical prophylaxis: (a) the agents should be delivered to the surgical site prior to the initial incision, and (b) bactericidal antibiotic concentrations should be maintained at the surgical site throughout the surgical procedure. Although animal and human models have demonstrated the efficacy of a single dose of an antibiotic administered just prior to bacterial contamination, long operations often require intraoperative doses of antibiotics to maintain adequate concentrations at the surgical site for the duration of surgery.31 Antibiotics should be administered with anesthesia just prior to the initial incision. Administration of antibiotics too early may result in concentrations below the MIC toward the end of the operation, and administration too late leaves the patient unprotected at the time of initial incision. In a study examining the timing of antibiotic administration to 2,847 patients receiving prophylaxis, Classen et al.31 evaluated patients who received prophylaxis early (2 to 24 hours before surgery), preoperative prophylaxis (0 to 2 hours prior to surgery), perioperative prophylaxis (up to 3 hours after first incision), and postoperative prophylaxis (>3 hours after the first incision). The risk of infection was lowest (0.6%) for patients who received preoperative prophylaxis, moderate (1.4%) for those who received perioperative antibiotics, and greatest for those who received postoperative antibiotics (3.3%) or preoperative antibiotics too early (3.8%). The risk for an SSI increases dramatically with each hour from the time of initial incision to the time when antibiotics are eventually administered. For these reasons, prophylactic antibiotics should not be prescribed to be given “on call to the operating room (OR),” which can occur two or more hours prior to the initial incision, nor should concurrent therapeutic antibiotics be relied on to provide adequate protection. In both situations, the chance for improperly timed doses is high. Although the landmark study by Classen et al.31 confirmed that antimicrobial prophylaxis should be administered within 2 hours prior to the initial incision, administration immediately prior to the incision may not allow enough time for the drug to distribute throughout the tissues involved in the surgery.
In a large prospective observational study of 3,836 visceral, trauma, and vascular surgeries where antimicrobial prophylaxis with cefuroxime and metronidazole was employed, the incidence of SSIs was analyzed according to the timing of antimicrobial administration. When antimicrobial prophylaxis was administered within 30 minutes or between 1 and 2 hours before the initial incision, the risk of SSI was greater when compared to antimicrobial prophylaxis administered 30 to 59 minutes prior to the initial incision. The authors conclude that the optimal window for antimicrobial (cefuroxime and metronidazole) is between 30 and 59 minutes prior to the initial incision.32 This effect may be a function of the pharmacodynamics and pharmacokinetics of the antimicrobial chosen for the prophylactic regimen. A larger study of 4,472 patients undergoing cardiac, orthopedic, and gynecologic surgery with a variety of antimicrobial prophylactic regimens also evaluated the temporal relationship between SSI occurrence and the timing of antibiotics. After excluding patients who received drugs with prolonged infusion times (i.e., fluoroquinolones and vancomycin), there was a statistically nonsignificant trend toward fewer SSIs in patients who received their prophylactic regimen within the 30 minutes prior to incision as compared with those who received the regimen 31 to 60 minutes prior to incision (odds ratio, OR: 1.74; 95% confidence interval: 0.98 to 3.04).33
Despite the importance of appropriately timed prophylactic antibiotic therapy, few patients receive antibiotics at the optimal time in relation to surgery. Potential barriers include antibiotics ordered after the patient has arrived in the OR, delayed antibiotic preparation or delivery, and use of antibiotics that require long infusion times. One study assessed the timing of prophylactic antibiotics in 100 patients and found that only 26% of patients received an antibiotic dose within 2 hours of the initial surgical incision.34
Although most studies comparing single versus multiple doses of prophylactic antibiotics have failed to show a benefit of multidose regimens, the duration of operations in these studies may not be as long as that frequently observed in clinical practice. Proponents of administering a second antibiotic dose during lengthy operations suggest that the risk for SSI is just as great at the end of surgery (during wound closing) as it is during the initial incision. One study of patients undergoing clean–contaminated operations suggests that procedures longer than 3 hours require a second intraoperative dose of cefazolin or substitution of cefazolin with a longer-acting antimicrobial agent.4 A second study of patients undergoing elective colorectal surgery suggests that low serum antimicrobial concentrations at the time of surgical closure is the strongest predictor of postoperative SSI.35 Studies of patients undergoing cardiac surgery also have demonstrated a higher infection rate among patients with undetectable antibiotic serum concentrations at the conclusion of the procedure.36
One strategy to ensure appropriate redosing of prophylactic antibiotics during long operations is use of a visual or auditory reminder system. One hospital reported its experience with such a system, finding that an automated reminder improved compliance and reduced SSIs. However, even with the reminder system, intraoperative redosing was done in only 68% of eligible patients.37 Another strategy currently being evaluated is the role of continuous infusions of cefazolin, which one pilot study has found to be a feasible way to ensure adequate serum concentrations of antibiotic during prolonged surgeries.38 Further trials are required before such an intervention can be recommended.
ANTIMICROBIAL CHOICE
![]() The choice of prophylactic antibiotic depends on the type of surgical procedure, the most frequent pathogens seen with this procedure, safety and efficacy profiles of the antimicrobial agent, current literature evidence supporting its use, and cost. Although most SSIs involve the patient’s normal flora, antimicrobial selection also must take into account the susceptibility patterns of nosocomial pathogens within each institution. Typically, gram-positive coverage should be included in the choice of surgical prophylaxis because organisms such as S. aureus and S. epidermidis are encountered commonly as skin flora. The decision to broaden antibiotic prophylaxis to agents with gram-negative and anaerobic spectra of activity depends on both the surgical site (e.g., upper respiratory, GI, or genitourinary tract) and whether the operation will transect a hollow viscous or mucous membrane that may contain resident flora.3
The choice of prophylactic antibiotic depends on the type of surgical procedure, the most frequent pathogens seen with this procedure, safety and efficacy profiles of the antimicrobial agent, current literature evidence supporting its use, and cost. Although most SSIs involve the patient’s normal flora, antimicrobial selection also must take into account the susceptibility patterns of nosocomial pathogens within each institution. Typically, gram-positive coverage should be included in the choice of surgical prophylaxis because organisms such as S. aureus and S. epidermidis are encountered commonly as skin flora. The decision to broaden antibiotic prophylaxis to agents with gram-negative and anaerobic spectra of activity depends on both the surgical site (e.g., upper respiratory, GI, or genitourinary tract) and whether the operation will transect a hollow viscous or mucous membrane that may contain resident flora.3
Although antimicrobial prophylaxis can be administered through a variety of routes (e.g., oral, topical, or intramuscular), the parenteral route is favored because of the reliability by which adequate tissue concentrations may be acheived.39 Cephalosporins are the most commonly prescribed agents for surgical prophylaxis because of their broad antimicrobial spectrum, favorable pharmacokinetic profile, low incidence of adverse side effects, and low cost. First-generation cephalosporins, such as cefazolin, are the preferred choice for surgical prophylaxis, particularly for clean surgical procedures.3,4,7 In cases where broader gram-negative and anaerobic coverage is desired, antianaerobic cephalosporins, such as cefoxitin and cefotetan, are appropriate choices. Although third-generation cephalosporins (e.g., ceftriaxone) have been advocated for prophylaxis because of their increased gram-negative coverage and prolonged half-lives, their inferior gram-positive and anaerobic activity and high cost have discouraged the widespread use of these agents.3,4,7
Allergic reactions are the most common side effects associated with cephalosporin use. Reactions can range from minor skin manifestations at the site of infusion to rash, pruritus, and rarely anaphylaxis (<0.02%). The structural similarity between penicillins and cephalosporins (each contains a β-lactam ring) has led to considerable confusion about the cross-allergenicity between these two classes of drugs. Twenty percent of the general population is labeled “penicillin allergic,” yet of these patients, only 10% to 20% have positive results of a penicillin skin test.40 The rate of cross-reactivity is –2%, but as only 20% of all “penicillin-allergic” patients truly are penicillin allergic, the true incidence of cross-reactivity likely is less than 1%. Routine penicillin skin testing is not cost-effective.40 In summary, the administration of cephalosporins is both safe and cost-effective for many patients who are labeled “penicillin allergic,” and they can be used by patients who have not experienced an immediate or type I penicillin allergy.
Vancomycin can be considered for prophylactic therapy in surgical procedures involving implantation of a prosthetic device in which the rate of MRSA is high.23,41 If the risk of MRSA is low, and a β-lactam hypersensitivity exists, clindamycin can be used for many procedures instead of cefazolin to limit vancomycin use. Infusion-related side effects, such as thrombophlebitis and hypotension, particularly with vancomycin, usually can be controlled by adequate dilution and slower administration rates.42
Pseudomembranous colitis secondary to cephalosporins is uncommon and generally easily treated with a short course of oral metronidazole. Although infrequent, bleeding abnormalities related to cephalosporin use have been reported.43 The primary hematologic effect appears to be inhibition of vitamin K-dependent clotting factors that results in prolongation of the prothrombin time. The mechanism for this effect, most commonly seen with cefotetan, is related to the methylthiotetrazole side chain of the β-lactam molecule. Patients at greatest risk for this hypoprothrom-binemic effect have received a prolonged course of these agents and have underlying risk factors for vitamin K deficiency, such as malnutrition.44
Because inappropriate prophylactic antibiotic use not only can induce antibiotic resistance but also can negatively affect an institution’s antibiotic budget, initiatives to curtail inappropriate antibiotic use have become the focus of many drug use evaluation efforts. Potential sources of inappropriate antibiotic prophylaxis include the use of broad-spectrum antimicrobials when a narrow-spectrum agent is warranted, extending prophylaxis for durations beyond that recommended in published guidelines, and using expensive antibiotics when equivalent, less expensive agents are available. The most effective tools for ensuring appropriate prophylactic antibiotic prescribing are knowledge of the institutional postoperative infection rate for each type of surgical procedure and familiarity with the bacterial epidemiology patterns for each surgical population. Individualized institutional guidelines that take into account the best literature evidence, institution-based antibiotic susceptibility data, and surgeon preference are important tools for rationalizing antibiotic prophylaxis use.45
RECOMMENDATIONS FOR SPECIFIC TYPES OF SURGERY
Guidelines for surgical prophylaxis usually are structured according to the tissues affected during an operation. Although many different surgical procedures may be performed at any one anatomic site, this method of categorization still is optimal because the factors related to the success of a prophylactic regimen, such as the endogenous flora that are expected and the pharmacokinetics, pharmacodynamics, and spectrum of selected antimicrobials, generally are constant for a particular surgical site (see the discussion above). The choice of antimicrobial prophylaxis is always best evaluated using the results of properly conducted clinical trials. In the absence of studies specific to the procedure in question, extrapolation from data on regimens for different procedures in the same anatomic site in question usually can be made. Subsequent modifications to each prophylactic regimen should be based on intraoperative findings or events.
![]() A comprehensive review of the surgical prophylaxis literature is beyond the scope of this chapter, but important factors are reviewed here for each type/site of surgery. Specific recommendations are summarized in Table 101-6. The reader is referred to published guidelines and review articles.2,3,4,7,39,46,47
A comprehensive review of the surgical prophylaxis literature is beyond the scope of this chapter, but important factors are reviewed here for each type/site of surgery. Specific recommendations are summarized in Table 101-6. The reader is referred to published guidelines and review articles.2,3,4,7,39,46,47
TABLE 101-6 Most Likely Pathogens and Specific Recommendations for Surgical Prophylaxis
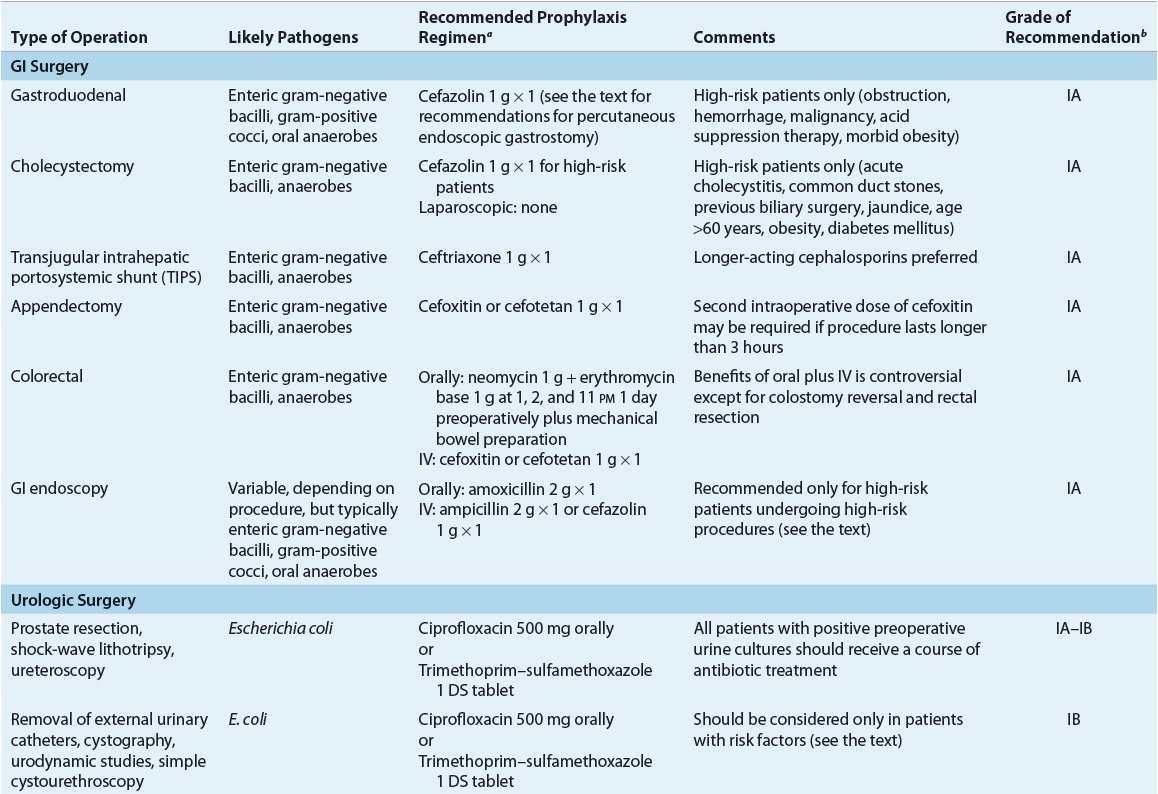
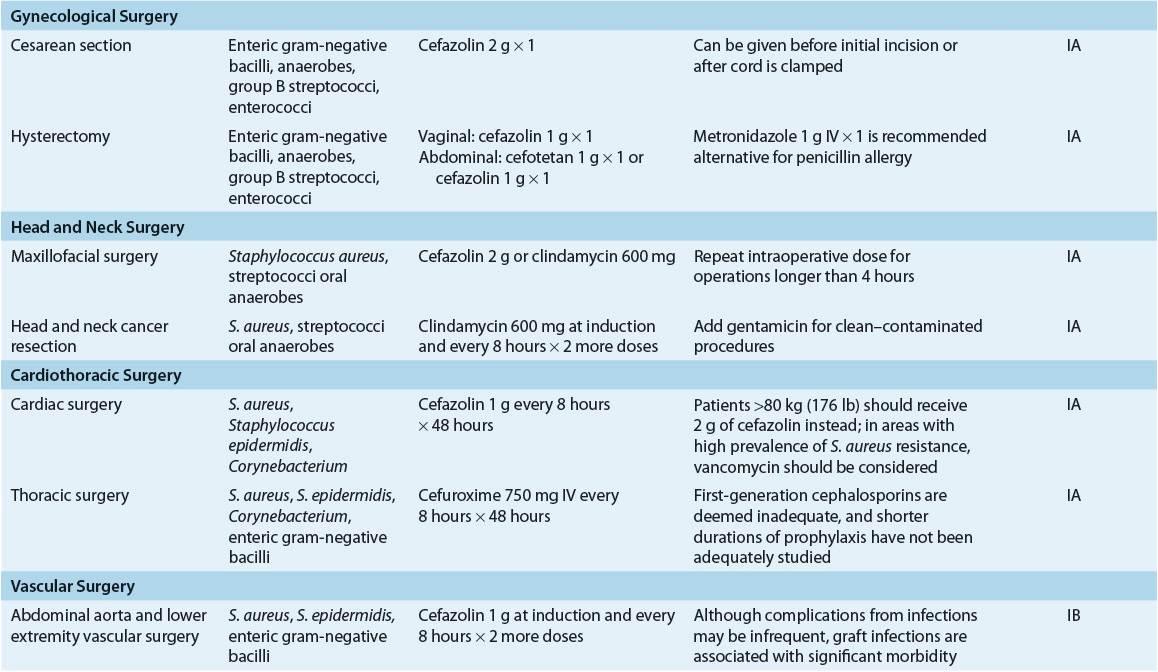
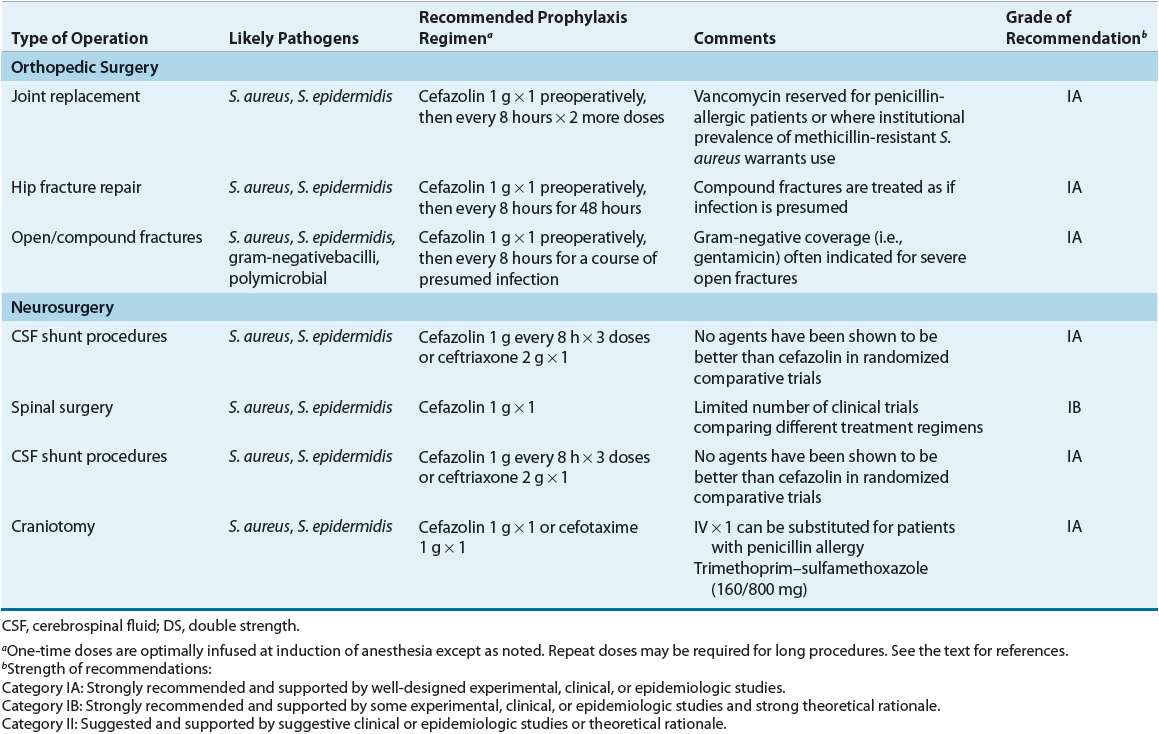
Gastrointestinal Surgery
GI surgery can be categorized according to surgical site and infectious risk. Gastroduodenal surgery and hepatobiliary surgery generally are considered to be clean or clean–contaminated surgeries, with SSI rates generally less than 5%. Colorectal surgery, including appendectomies, is considered contaminated because of the large quantities and polymicrobial nature of bacterial flora within the colon. SSI rates for these types of surgeries generally range from 15% to 30%. Emergent abdominal surgery involving bowel perforation or peritonitis is considered a dirty surgical procedure, associated with a greater than 30% risk of SSI, and should be treated with therapeutic rather than prophylactic antibiotics.3
Gastroduodenal Surgery
Insignificant numbers of bacteria usually are found in the stomach and duodenum because of their acidity. The rate of SSIs in gastroduodenal surgery generally is low, so procedures in this region can be classified as clean. The risk for an SSI in this population increases with any condition that can lead to bacterial overgrowth, such as obstruction, hemorrhage, or malignancy, or increasing the pH of gastroduodenal secretions with concomitant acid suppression therapy. Antimicrobial prophylaxis is of clinical benefit only in this high-risk population. In most cases, a single dose of IV cefazolin will provide adequate prophylaxis.48 For patients with a β-lactam allergy, oral ciprofloxacin is as efficacious as parenteral cefuroxime as prophylactic therapy for gastroduodenal surgery.48 Antimicrobial prophylaxis is indicated in esophageal surgery only in the presence of obstruction. Postoperative therapeutic antibiotics may be indicated if perforation is detected during surgery, depending on whether an established infection is present.
Use of antibiotic prophylaxis for percutaneous endoscopic gastrostomy placement is controversial.49 Although postoperative peristomal infection can occur in up to 30% of patients, clinical trials with cefazolin given 30 minutes preoperatively in this population are conflicting.49 A pharmacoeconomic study that incorporated a meta-analysis of available studies to determine efficacy suggested that antibiotic prophylaxis was cost-effective for patients undergoing percutaneous endoscopic gastrostomy placements.50
Hepatobiliary Surgery
Although bile normally is sterile, and the SSI rate after biliary surgery is low, antibiotic prophylaxis is of benefit in this population. Bile contamination (bactobilia) can increase the frequency of SSIs and is present in many patients (e.g., those with acute cholecystitis or biliary obstruction and those of advanced age).46 In general, however, the correlation between bactobilia in surgical specimens and the subsequent pathogens implicated in an SSI is poor. The most frequently encountered organisms are E. coli, Klebsiella species, and enterococci. Pseudomonas is an uncommon finding in the absence of cholangitis. Trials comparing first-, second-, and third-generation cephalosporins have not demonstrated benefit over single-dose cefazolin prophylaxis even in high-risk patients (e.g., age >60 years, previous biliary surgery, acute cholecystitis, jaundice, obesity, diabetes, and common bile duct stones).51 Ciprofloxacin and levofloxacin are effective alternatives for β-lactam-allergic patients undergoing open cholecystectomy.52,53 In fact, orally administered levofloxacin appears to provide similar intraoperative gallbladder tissue concentrations.53 For low-risk patients undergoing elective laparoscopic cholecystectomy, antibiotic prophylaxis is not of benefit and is not recommended.54 The risk for SSIs in cirrhotic patients undergoing transjugular intrahepatic portosystemic shunt surgery may be reduced with a single prophylactic dose of ceftriaxone,55 but not with single doses of shorter-acting cephalosporins.56
Although surgeons may use presumptive antibiotic therapy for patients with acute cholecystitis or cholangitis and defer surgery until the patient is afebrile in an effort to decrease the risk of subsequent infections, this practice is controversial. Detection of an active infection during surgery (e.g., gangrenous gallbladder and suppurative cholangitis) is an indication for a course of postoperative therapeutic antibiotics. In either case, antibiotics with additional antianaerobic activity (e.g., cefoxitin or cefotetan) are indicated.57
Appendectomy
Suspected appendicitis is a frequent cause of abdominal surgery. Numerous antibiotic regimens, all with activity against gram-positive and gram-negative aerobes and anaerobic pathogens, are effective in reducing SSI incidence.46 A cephalosporin with antianaerobic activity, such as cefoxitin or cefotetan, is recommended as first-line therapy; however, a comparative trial of cefoxitin and cefotetan suggests that cefotetan may be superior, possibly because of its longer duration of action.58 In patients with β-lactam allergy, metronidazole in combination with gentamicin is an effective regimen. Broad-spectrum antibiotics covering nosocomial pathogens (e.g., Pseudomonas) do not further reduce SSI risk and instead may increase the cost of therapy and promote bacterial resistance.59 Although single-dose therapy with cefotetan is adequate, prophylaxis with cefoxitin may require intraoperative dosing if the procedure extends beyond 3 hours. Established intraabdominal infections (e.g., gangrenous or perforated appendix) require an appropriate course of postoperative therapeutic antibiotics. Laparoscopic appendectomy produces lower postoperative infection rates than open appendectomy; however, antimicrobial prophylaxis was used for all patients in these studies; thus, the role for prophylaxis in this population remains poorly studied.60
Colorectal Surgery
In the absence of adequate prophylactic therapy, the risk for SSI after colorectal surgery is high because of the significant bacterial counts in fecal material present in the colon (frequently >109 per gram). Anaerobes and gram-negative aerobes predominate, but gram-positive aerobes also may play an important role. Reducing this bacterial load with a thorough bowel preparation regimen (4 L of polyethylene glycol solution or 90 mL of sodium phosphate solution administered orally the day before surgery) is controversial; however, 99% of surgeons in a survey routinely use mechanical preparation.61 Risk factors for SSIs include age over 60 years, hypo-albuminemia, poor preoperative bowel preparation, corticosteroid therapy, malignancy, and operations lasting longer than 3.5 hours.7
Antimicrobial prophylaxis reduced mortality from 11.2% to 4.5% in a pooled analysis of trials comparing antimicrobial prophylaxis with no prophylaxis for colon surgery.62 Effective antibiotic prophylaxis reduces even further the risk for an SSI. Several oral regimens designed to reduce bacterial counts in the colon have been studied.46 The combination of 1 g neomycin and 1 g erythromycin base given orally 19, 18, and 9 hours preoperatively is the regimen most commonly used in the United States.63 Neomycin is poorly absorbed, but provides intraluminal concentrations that are high enough to effectively kill most gram-negative aerobes. Oral erythromycin is only partially absorbed but still produces concentrations in the colon that are sufficient to suppress common anaerobes. If surgery is postponed, the antibiotics must be readministered to maintain efficacy. Optimally, the bowel preparation regimen should be completed prior to starting the oral antibiotic regimen. This is of particular concern because most procedures now are performed electively on a “same-day surgery” basis. In this case, the bowel preparation regimen is self-administered by the patient at home on the day prior to hospital admission, and compliance cannot be monitored carefully.
Patients who cannot take oral medications should receive parenteral antibiotics. Cefoxitin or cefotetan is used most commonly, but other second- and some third-generation cephalosporins also are effective.64 The role of metronidazole in combination with cephalosporin therapy is unclear. Only retrospective evidence suggests that the addition of metronidazole to a cephalosporin or extended-spectrum penicillin provides additional benefit.65 Until this finding is confirmed in prospective studies, metronidazole should be reserved for combination therapy with cephalosporins with poor anaerobic coverage (e.g., cefazolin). At this time, the evidence recommending the addition of metronidazole to cephalosporins with anaerobic activity (e.g., cefotaxime, cefoxitin, and ceftriaxone) is insufficient.66 For β-lactam-allergic patients, perioperative doses of gentamicin and metronidazole have been used. Combination therapy (i.e., oral and IV therapy) is controversial. A Cochrane review suggests that combination therapy is superior to either oral or IV antibiotics alone.66 However, the largest study (491 patients) comparing combination therapy with only IV therapy, which showed no benefit with combination therapy, was not included in the meta-analysis.67 Postoperative antibiotics generally are unnecessary in the absence of any untoward events or findings during surgery. IV antibiotics are required for colostomy reversal and rectal resection because enterally administered antibiotics will not reach the distal segment that is to be reanastomosed or resected.68



