Antimicrobial Dosing in Obesity
Alexander H. Flannery, Aaron M. Cook, and Craig A. Martin
Outline
•Introduction
•Antibiotics
•Antifungals
•Antivirals
•Sample Calculations
•Summary Table: Antimicrobial Drugs
Managing antimicrobial dosing in obesity can be quite a challenge for clinicians across the spectrum of care. Not only does underdosing patients risk therapeutic failure, but it also has the potential to induce antimicrobial resistance, exposing the microorganism to subtherapeutic concentrations of the drug. In high doses, however, some antimicrobials may be fairly toxic and adverse events may occur if total body weight (TBW) is used for all drugs. As we begin to discuss the dosing of antimicrobials in obesity, let us briefly review the optimization of pharmacokinetic/pharmacodynamic (PK/PD) targets and the impact that alterations in obesity may have on reaching these PK/PD targets.
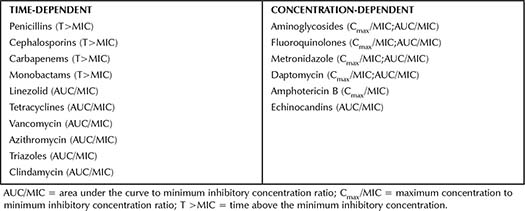
Activity for antimicrobials can generally be divided into two broad classifications: time-dependent or concentration-dependent. Within those classifications, PK/PD targets that best correlate with efficacy are unique for each antimicrobial class. For example, beta-lactams are optimized when the concentration of the free drug remains above the minimum inhibitory concentration (MIC). In contrast, aminoglycosides are concentration-dependent with dose optimization that targets the maximum concentration (Cmax) over the MIC (or Cmax/MIC) and total area under the curve (AUC), AUC/MIC. Table 2-1 lists PK/PD targets for commonly used antimicrobials.1-3
Table 2-1. Activity In Vitro and PK/PD Measures Best Correlated with Efficacy for Commonly Used Antimicrobials
As discussed in Chapter 1, obesity can alter a number of PK parameters. Applying these changes to the principles of achieving PK/PD targets, one can easily see the importance of these PK changes in obesity. For example, do the changes in the volume of distribution (Vd) that occur in obesity influence the Cmax of the drug in question? Does the possibility of increased clearance in obesity reduce the time above the MIC (T >MIC) for a time-dependent antibiotic? The task of the clinician dosing antimicrobials in obesity is to identify dosing strategies that optimize these targets, yet keep drug levels within a safe range without predisposing to adverse effects.
This chapter reviews antimicrobial dosing in three major sections, covering antibiotics, antifungals, and antivirals all broken down into classes. In some cases, fairly consistent opinion exists of the alterations necessary to an antimicrobial regimen in obesity. Unfortunately, many antimicrobial agents may lack human or animal data regarding dosing in obesity. In these cases, we have provided as evidence-based rationale as possible for our recommendations where applicable in these scenarios. The ultimate decision of dosing in obesity must include a careful assessment by the clinician of the institution’s antibiogram, MIC of the infecting organism, clinical condition of patient, and ultimately, a risk-benefit assessment with a careful monitoring plan in place for therapeutic efficacy and safety. (See Summary Table: Antimicrobial Drugs.)
Antibiotics
Beta-lactams
The beta-lactams consist of several classes of structurally related agents including the penicillins, cephalosporins, carbapenems, and monobactams. All of these agents are weak organic acids and, with a few exceptions, are relatively hydrophilic in vivo. As such, the Vd tends to correlate to TBW or lean body mass in most individuals (likely until reaching the extremes of obesity). Beta-lactams such as nafcillin and ceftriaxone exhibit moderately high protein binding due to their relative lipophilicity. The Vd of these agents has not been well described in obese individuals; however, it would be expected to be higher than in normal-weight individuals and partially dependent on the available fraction of unbound drug.
Piperacillin has been the most well-studied penicillin medication in the obese population, and the appropriate dose appears to be similar to normal-weight patients. Two separate PK studies employed Monte Carlo simulation to estimate the probability of target attainment and suggested that conventional doses of piperacillin (and tazobactam) may not reliably achieve PD targets (although loss of target attainment was generally seen above an MIC of 8 to 16 mcg/mL, which is typically the susceptibility threshold).4,5 Sturm et al. also demonstrated that the Vd was approximately 400% higher than that of a normal-weight individual.5 Another Monte Carlo analysis of the obese individuals in the Sturm and Cheatham reports was performed by the same group and indicated that piperacillin clearance is significantly associated with body mass index (BMI), and Vd was associated with TBW.6 Prolonged infusion of typical piperacillin/tazobactam doses were recommended. Two separate case reports of very obese males (220 kg and 167 kg) investigated piperacillin PKs and corroborated that the Vd in an individual patient was approximately 400% of normal-weight individuals.7,8 The two case reports showed somewhat conflicting data on clearance, with one describing a clearance rate of approximately 240% and the other demonstrating a half-life that was 200% of the usual half-life in a normal-weight patient.7,8 Finally, a retrospective analysis of prospectively collected piperacillin concentrations in critically ill obese patients suggested that piperacillin PK parameters were similar to critically ill nonobese patients, although considerable variability was evident.9 Doses at the higher end of the normal dosing range may be more appropriate for obese individuals with normal renal function. Therapeutic drug monitoring in obese patients receiving piperacillin, particularly those who are critically ill or have pathogens near the susceptibility breakpoint, should be considered.10
Despite the long-standing availability of the penicillin products ranging from penicillin G to aminopenicillins (ampicillin and amoxicillin) to carboxypenicillins (ticarcillin), little to no data exist regarding the impact of obesity on the PKs of these agents. Although agents such as ampicillin, ampicillin/sulbactam, penicillin, and ticarcillin have not been rigorously studied in obese individuals, based on the PKs of these agents it is likely that each of these agents would exhibit similar alterations in PKs compared to piperacillin. It would be expected that these agents would exhibit a larger Vd, perhaps necessitating larger than normal doses (or at minimum, doses on the high end of the typical dose range) at a similar or more frequent interval.
The primary role of beta-lactams, particularly cephalosporins, in surgical infection prophylaxis has provided a good model for dosing in obese individuals, particularly with bariatric surgery. Several factors impact the PK parameters in the acute perioperative setting including blood loss, tissue perfusion, and systemic inflammation. Tissue penetration for these agents appears to be adequate with standard dosing in most studies, although one study of obese patients undergoing abdominal and pelvic surgery suggested that cefoxitin tissue concentrations were below the MIC for typical pathogens.11 In this PK analysis, obese patients exhibited a lower peak concentration (normalized to the dose given), and the Vd correlated significantly with lean body mass. A similar study investigating cefazolin PKs during caesarean delivery also found suboptimal tissue concentrations (approximately 33% less than normal-weight individuals) and suggested that cefazolin tissue concentrations had a significant inverse relationship with BMI.12 Use of higher doses of cefazolin (3 g) for surgical prophylaxis may not yield significantly higher adipose tissue concentrations.13 Cefotetan PKs in obese individuals undergoing colorectal surgery were not reported, but patients with increased BMI also had an increased risk of infection, perhaps suggesting inadequate antimicrobial exposure.14
Very few studies investigating cephalosporin PKs in obese, ambulatory, and nonsurgical subjects have been published. Obese and normal-weight volunteers receiving cefotaxime were compared in a small PKs study.15 The investigators found that although the half-life and Vd of cefotaxime was similar between groups, the clearance varied greatly. Total body clearance ranged from 20% lower in obese patients overall to 25% higher in cases of extreme obesity. Ceftaroline has exhibited similar behavior in obese individuals, suggesting up to 30% lower plasma concentrations compared to normal-weight individuals, yet outcome studies compared to nonobese individuals fail to show a difference in skin and soft tissue infections.16,17 Although agents such as cefuroxime, cefdinir, ceftazidime, and ceftriaxone have not been rigorously studied in obese individuals, based on the PKs of these agents it is likely that each of these agents would exhibit similar alterations in PKs compared to other cephalosporins. It would be expected that these agents would exhibit a larger Vd, perhaps necessitating larger than normal doses (or at minimum, doses on the high end of the typical dose range) at a similar or more frequent interval to optimize tissue concentration, particularly in the context of surgery or in the treatment of serious infections.
Carbapenems such as imipenem and ertapenem have been studied to a limited extent in obese patients. Ertapenem PKs in obese individuals were compared to normal-weight volunteers. This study demonstrated a 17% reduction in AUC in patients with BMI >40 kg/m2, likely due in part to a 40% increase in Vd.18 Similar to cephalosporins, many of the studies investigating carbapenem PKs have been done in patients undergoing surgery (primarily colorectal or bariatric), although the impact of obesity on carbapenem PKs is conflicting. One study investigating ertapenem PKs in bariatric surgery patients showed that the plasma concentrations and clearance were comparable to normal-weight individuals.19 However, the AUC in the morbidly obese population was approximately 20% less than normal-weight individuals. Monte Carlo simulation suggested that standard dosing of ertapenem (1 g) may not be adequate to achieve goal concentrations in obese patients.18 Other clinical studies have corroborated these results, suggesting that lower ertapenem exposure may lead to increased incidence of surgical wound infection, although carbapenems may be less associated with failure in obese patients than cephalosporins.14,20
Other carbapenems such as meropenem have been studied sparsely. Meropenem was shown to have a comparable peak concentration and clearance in morbidly obese, intensive care unit (ICU) patients and in obese patients with variable renal function compared to normal-weight individuals.21,22 The Vd when normalized by weight was slightly less in obese individuals, which suggests incomplete distribution into adipose tissue. Overall, doripenem Vd is higher in obese patients than in normal-weight individuals (83%), although volume normalized by weight is lower (similar to meropenem).23 Monte Carlo simulation suggested that standard dosing of doripenem or meropenem may be adequate to achieve goal concentrations in obese patients for pathogens with lower MICs, but it did not achieve reliable target attainment against isolates such as Acinetobacter species.23 Higher doses or alternative agents may be necessary in this clinical setting. Limited information is available regarding the monobactams, but given the similarity to the compounds above, we provide the same overall guidance when considering aztreonam dosing in obesity.
Helpful Tips 
•When using beta-lactams to treat serious infections, consider the use of a loading dose followed by a continuous or extended infusion to optimize the PK/PD parameters of these agents.
•The increase in Vd of beta-lactams may contribute to reduced overall exposure, although the evidence to support higher dosing is limited. Given the safety profile of beta-lactams, dosing on the higher end of the dosing range should be considered in the morbidly obese and when treating serious infections.
•Therapeutic drug monitoring in this population may be advisable when available, particularly in obese, critically ill patients.
Aminoglycosides
The aminoglycoside antimicrobials are hydrophilic weak bases, which are present in cationic form in vivo. This leads to a relatively low Vd approximating blood volume. In obese individuals, it is suggested that aminoglycosides incompletely distribute into the excess adipose mass, and an adjusted body weight is commonly used to account for this. Due to the PD factors that govern bacterial killing for aminoglycosides, these agents have optimal bactericidal activity when achieving a high peak concentration (commonly 8× to 10× the MIC of the targeted pathogen). To achieve target serum concentrations, accurate assessment of the adjusted body weight and optimization of the peak concentration is essential when using aminoglycosides in obese patients.
The Vd of aminoglycosides such as amikacin, gentamicin, and tobramycin in obese individuals has been described in several studies.24-28 The calculated Vd for gentamicin and tobramycin in obese individuals after receiving a dose of 1 mg/kg intravenously (IV) was larger than Vd in normal-weight individuals receiving the same weight-based dose. However, the increase in Vd was not proportional to the difference in weight between the two groups, suggesting that a correction factor is necessary to better approximate Vd in the obese population. The primary supposition is that adipose tissue contains 45% to 50% as much extracellular fluid as does nonadipose tissue; thus, the correction factor should approximate this percentage for calculating an adjusted body weight.29 This hypothesis has been corroborated by several PK studies that estimated adipose uptake of aminoglycoside agents ranging from 30% (gentamicin), 38% (amikacin), 43% (gentamicin), and 58% (tobramycin).24-27,30,31 Very little data exist regarding obese patients receiving extended-interval aminoglycosides. It is likely that the Vd will require similar adjustment for excess adipose mass, but use of the adjusted body weight is essentially an extrapolation from the data described above, which generated from more conventional dosing, and it should be used with caution. See Equation 1-6, Table 1-1, in Chapter 1: Introduction to Dosing Medications in Obese Patients.
Aminoglycosides are primarily eliminated unchanged in the urine, typically via filtration. Renal clearance of aminoglycosides in obese individuals appears to be similar to normal-weight subjects. A classic study investigating the PKs of amikacin in obese gastric-bypass patients demonstrated a half-life of 2.1 hours, which is comparable to that seen in normal-weight individuals.24 Similar PK studies evaluating gentamicin and tobramycin clearance in obese patients also suggest the elimination half-life in obese individuals is not significantly different than those with normal weight.26,29 Thus, ideal body weight (IBW) appears to be the best weight to use when estimating creatinine clearance and dosing interval, whereas adjusted body weight is most appropriate for calculation of the actual dose.
Helpful Tips 
•Therapeutic drug monitoring is readily available for aminoglycosides. Particularly if doing once-daily aminoglycoside dosing, consider levels following the first dose to calculate the patient’s true kinetics rather than relying on population estimates.
•For estimation of aminoglycoside clearance, use IBW in estimation equations for creatinine clearance.
•For loading and maintenance doses of aminoglycosides, use the adjusted body weight equation (see Equation 1-6, Table 1-1, in Chapter 1: Introduction to Dosing Medications in Obese Patients).
Glycopeptides and Daptomycin
Vancomycin and other glycopeptide antimicrobials, such as teicoplanin, oritavancin, and dalbavancin, are hydrophilic molecules with a high molecular weight. Despite the size and propensity of these agents to hydrogen bond to multiple sites, their Vd is greater than that approximated by blood volume. The Vd appears to correlate reasonably well with TBW (r2 = 0.66) or adjusted body weight (correction factor 0.4, r2 = 0.49).32 Earlier PK data suggest an adjusted body weight using a correction factor of 0.15 of TBW best approximates Vd.33,34 Currently, TBW is typically recommended when dosing glycopeptides such as vancomycin, although usually at a lower dose (10 mg/kg rather than 15 mg/kg) to avoid achieving elevated peak concentrations. One group has developed a divided loading dose strategy for vancomycin in obese patients specifically, with excellent goal trough achievement at 12 and 24 hours.35
Conflicting evidence exists defining the relationship of obesity and vancomycin clearance. Although some studies have demonstrated an increased vancomycin clearance in obese individuals, extremes of obesity may be associated with reduced clearance than less obese patients.32 Leong and colleagues completed a retrospective study evaluating the predictive value of various weights for estimating vancomycin clearance. In their study, adjusted body weight using the Leonard-Boro equation for vancomycin clearance was the most precise in predicting actual vancomycin concentrations.36 However, due to some of the assumptions related to the calculation of PK parameters, others have questioned the data.37 Prospective PK analysis demonstrated that vancomycin Vd is best associated with TBW (r2 = 0.86).34 The relatively common practice of dose capping vancomycin at 2 g per dose does not seem to be supported by the current literature, although patients with extreme obesity or low renal clearance are often underrepresented. Given the relative convenience of serum monitoring in most institutions, it is advisable to obtain serum concentrations to evaluate the vancomycin clearance and estimate overall drug exposure.
Daptomycin is a large molecule with hydrophilic regions, which might theoretically limit the Vd. However, TBW appears to be the most appropriate weight to estimate Vd for daptomycin in obese patients (r2 = 0.451).38,39 Daptomycin clearance is primarily renal; however, little is known about the precise effects of obesity on daptomycin clearance, although clearance appears to be higher in obese individuals compared to those of normal weight.38 Daptomycin AUC in obese subjects is approximately 30% higher than in nonobese controls.38
Helpful Tips 
•Be cautious regarding the use of TBW in dosing vancomycin in morbidly obese patients and in obese patients with lower rates of creatinine clearance.
•With therapeutic drug monitoring readily available, checking two levels following the first dose or vigilantly checking troughs early in therapy may help to ensure adequate levels while minimizing toxic exposures.
•Check a creatine phosphokinase level weekly with patients receiving daptomycin.
•Evidence suggests that vancomycin and daptomycin should be dosed on TBW in obesity.
Fluoroquinolones
The fluoroquinolone antimicrobials are amphiphilic agents that are water soluble but can still effectively penetrate lipid bilayers and adipose tissue. This leads to a relatively high Vd and extensive tissue penetration. In obese individuals, it is suggested that fluoroquinolones incompletely distribute into the excess adipose mass, and an adjusted body weight is commonly used to account for this.40
Following a single IV dose of 400 mg ciprofloxacin, the Vd was 23% greater and renal clearance was 29% greater in obese subjects compared to normal-weight controls, but it still yielded concentrations considered to be therapeutic.41 To normalize the Vd of obese subjects to normal-weight subjects, the authors suggest 45% of excess weight must be added to IBW; thus, the equation for adjusted body weight suggested in fluoroquinolone dosing41:
Equation 2-1: Adjusted body weight = IBW + 0.45 (TBW – IBW)
A case report of a patient with a BMI of 56.2 kg/m² receiving levofloxacin 4 mg/kg every 12 hours dosed on TBW reported the AUC was found to be double that of a normal-weight patient receiving a daily dose of 750 mg.42 A study in obese patients of a single dose of levofloxacin 750 mg IV demonstrated variable but relatively similar AUC values to that of nonobese patients.43 PKs of moxifloxacin have also been found to not be significantly affected in obesity.44 In a PK modeling study of patients with a BMI >40 kg/m², it was suggested that doses of levofloxacin in obese patients should be based more on renal function than weight. The group created a nomogram of doses ranging from 500 to 1,250 mg every 24 hours based on creatinine clearance using the Cockcroft-Gault equation (see Equation 1-8, Table 1-2, in Chapter 1: Introduction to Dosing Medications in Obese Patients) with IBW.45 It should be noted that one group studying an IV dose of ciprofloxacin 2.85 mg/kg dosed on TBW in obese and nonobese patients demonstrated a significantly elevated AUC; however, soft tissue concentrations were similar between the two groups, causing this group of authors to suggest that if not dosed on TBW, tissue penetration may be suboptimal.46 The implementation of these findings may be tempered by the consideration of very high-central compartment concentrations of fluoroquinolones, which may predispose to toxicities; thus, other drug choices may minimize adverse effects in these cases. Although some studies discussed above have used weight-based dosing of fluoroquinolones to achieve PK/PD targets, we caution readers with this approach as there is less clinical experience with weight-based fluoroquinolone dosing than the standard flat doses.
Helpful Tips 
•Do not forget that fluoroquinolones may prolong the QTc interval, particularly in patients on multiple QTc-prolonging medications.
Summary 
•Although some PK alterations likely exist in obesity, no changes are recommended in the standard doses of commonly used fluoroquinolones.
Oxazolidinones, Macrolides and Tetracyclines
Linezolid and tedizolid are oxazolidinone antimicrobials that effectively penetrate the bacterial cell wall to interact with the ribosome. Like fluoroquinolones, these agents are rather amphiphilic and tend to have a relatively high Vd and extensive tissue penetration. Conflicting evidence exists for linezolid on how to approach initial dosing. Clinicians may consider using the standard dose used in normal-weight individuals or applying an adjusted body weight to account for less efficient distribution into excess adipose mass. Linezolid clearance is best correlated to TBW, so independent of dose adjustment, the frequency of dosing may need to be increased in obese patients although current literature is conflicting. Several case reports or case series have demonstrated reduced linezolid concentrations in obese patients receiving standard linezolid dosing compared to nonobese patients secondary to an increased Vd; however, many of these reports still demonstrated favorable clinical outcomes.47-51 A PK study in healthy volunteers concluded that no dosage adjustment to the standard dose was necessary with patients weighing up to 150 kg.52 Some authors have suggested no changes to standard dosing below a BMI of 50 kg/m².53 In an analysis of data from two clinical trials comparing linezolid with vancomycin stratified by BMI quartiles, rates of clinical success were similar between obese and nonobese individuals receiving linezolid for nosocomial pneumonia and complicated skin infections.54 If using weight-based dosing of linezolid, some authors have suggested the use of an adjusted body weight.55 To deal with these PK alterations, some have recommended increasing the dosing interval. One group has even demonstrated subtherapeutic linezolid levels on a regimen of 600 mg IV every 8 hours (BMI = 72 kg/m²).56 Along with higher doses and potentially therapeutic drug monitoring, others have proposed the use of continuous infusion to achieve PK/PD targets.57 Considering the sum of the data and no impact on clinical outcomes with standard dosing in obesity, no dose adjustment is recommended empirically. However, when treating gram-positive pathogens that are less susceptible and with higher MICs or in extremes of obesity, consideration may be given to decreasing the dosing interval to every 8 hours. Although no data exist for tedizolid in obesity, given the similarity in chemical structures and extrapolating the data from linezolid, no empiric change in dose is recommended.
Despite their long-term, widespread use, limited evidence is available regarding the macrolides and tetracyclines. In clarithromycin-based regimens for Helicobacter pylori eradication, patients with a BMI of >25 kg/m² had lower rates of eradication compared to nonobese individuals.58 In a separate cohort of obese patients undergoing gastric bypass surgery treated for H. pylori with triple therapy including clarithromycin, a longer course was more effective than a short course.59 Although some have suggested this provides a rationale for higher dosing, it is impossible to distinguish whether the above results in H. pylori infection were due to PK changes in the macrolide component of the regimen, PK changes to other medications of the regimen, or the negative health consequences of obesity. In one case report, PKs of a 250-mg erythromycin base dose were examined pre- and postbariatric surgery in seven morbidly obese patients. Prior to bariatric surgery, the peak concentration observed in this cohort was similar to the peak concentration in nonobese individuals, leading some authors to suggest IBW if using weight-based dosing recommendations.60,61 It also is reasonable to conclude from the data that no change is recommended to the standard dosing of these agents when used in obesity.
Evidence is similarly limited for the tetracyclines. Body size has previously been linked as a pertinent covariate of tigecycline clearance in two population PK studies.62,63 In a recent observational study in critically ill patients with a variety of sites of infections, fewer successes with tigecycline were observed in obese patients, specifically those with a BMI >35 kg/m².64 These studies raise some concern that perhaps the lipophilic nature of tigecycline results in an increased clearance and thus a lower AUC/MIC ratio. However, in a PK study comparing the kinetics of tigecycline in class III obese and normal-weight, healthy volunteers given a single dose of 100 mg, the concentration-time profiles were remarkably similar between the two weight groups, suggesting that similar doses may be used in both obese and nonobese patients.65 Although doxycycline and minocycline are more lipophilic than tetracycline and likely influence drug dosing in obesity, limited information exists to guide any recommendations at this time.66
Helpful Tips 
•Consider severity of infection, and drug–drug interactions (particularly with selective serotonin reuptake inhibitors), prior to using higher than the standard total daily dose of linezolid.
•Do not forget that macrolides may prolong the QTc interval, particularly in patients on multiple QTc-prolonging medications.
•Although a broad-spectrum agent, tigecycline has a notable hole in coverage against Pseudomonas species.
Summary 
•No changes to the standard dosing of linezolid can be recommended based on limited evidence, although obese patients may demonstrate increased clearance.
•No changes to standard dosing of macrolides can be recommended, although the evidence for this class of medications is extremely limited in relation to the other medications discussed in this chapter.
•No dose adjustment is recommended for tigecycline. There is insufficient evidence to make a recommendation on tetracycline, doxycycline, and minocycline.
Polymyxins
The polymyxins have seen resurgence of late due to the development of multidrug-resistant, gram-negative organisms. Patients who receive these medications are usually receiving them as a last-line agent against multidrug-resistant organisms, and ensuring appropriate dosing to avoid resistance is vital. Additionally, these medications are potent nephrotoxins and dosed on body weight; therefore, identifying the appropriate weight to dose these medications on carries important safety implications.
The package insert recommends using IBW in weight-based dosing of colistin.67 Although some have contended, the published data align with the recommendation for IBW. In a retrospective cohort of 126 patients, nephrotoxicity was significantly associated with a higher colistin total daily dose (mg/kg of IBW per day).68 Although some have argued to use TBW in colistin dosing, the results from this study dispute that argument. Given the nephrotoxicity rates in the cohorts receiving 3 to 4.9 mg/kg IBW/day and ≥5 mg/kg IBW/day were 33% and 69%, respectively, TBW (or even adjusted body weight) may pose increased safety risks based on these study results.68 Increasing risks of nephrotoxicity with increasing doses (in mg/kg IBW/day) was also reported in a smaller retrospective cohort.69 Of particular mention, obese patients who received what the authors classified as excessive daily dosing (due to the fact that TBW was used) were 13.2 times more likely to develop nephrotoxicity than patients receiving normal or low-normal dosing.69 In a retrospective case-control study limited to overweight or obese patients (TBW >140% of their IBW), the rate of nephrotoxicity approached 50%, with a BMI >31.5 kg/m² an independent predictor of nephrotoxicity.70 The authors attributed the high degree of excessive dosing associated with using TBW to the high rate of nephrotoxicity in the cohort.70 In a prospective PK study of colistin in critically ill patients, the authors found that body weight was a covariate impacting the central volume of colistin methanesulfonate, suggesting that IBW be used for weight-based loading doses.71 In contrast, these authors proposed formulas for maintenance dosing that did not include weight, rather the target colistin steady-state concentration and CrCl.71 As new data continue to emerge, it appears if using a weight-based approach that IBW is supported by the most evidence at this time.
Compared with colistin, the information available regarding polymixin B dosing in obesity is scarce. Despite that colistin (polymyxin E) differs from polymyxin B by only one amino acid, data differ for the recommended dosing weight in obesity. The only available data regarding polymyxin B in obesity are from a prospective study of polymyxin B PKs in 24 critically ill patients.72 During modeling, TBW explained PK variability superior to IBW, thus driving the recommendation to use TBW for loading and maintenance doses of polymyxin B. Although this study had a single patient weighing 250 kg, it included no patients between 110 to 250 kg.72 Readers are advised to use caution in extremes of obesity.
Helpful Tips 
•Be extremely careful with dosing these agents. In the United States, colistin is dosed in terms of the base component (colistin), although doses are occasionally discussed in the literature in terms of the salt (colistimethate sodium).73 Additionally, both agents may be dosed in units or mg, often creating an error-prone situation.
Summary 
•IBW is recommended to dose colistin.
•
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





