and FJE Vajda2
(1)
Clinical Neurology and Neuropharmacology, University of Queensland, and Honorary Consultant Neurologist, Royal Brisbane and Women’s Hospital, Brisbane, QLD, Australia
(2)
Department of Medicine and Neurology Director of the Australian Epilepsy and Pregnancy Register, University of Melbourne and Royal Melbourne Hospital, Melbourne, Australia
Abstract
This chapter deals with two main issues, viz. the possible effects of pregnancy on the course of maternal epileptic seizure disorders and the effects of seizure disorders on pregnant women and their foetuses.
The little information that is available suggests that pregnancy tends to increase the risk of loss of seizure control in women whose epilepsy is untreated. The interpretation of the more extensive literature concerning antiepileptic drug-treated epilepsy in pregnancy is confounded by the effect of pregnancy in tending to decrease circulating concentrations of antiepileptic drugs relative to drug dose. It appears that, in the majority of instances, seizure control does not deteriorate during antiepileptic drug-treated pregnancy but that, if seizure control changes, it is more likely to worsen than to improve. However, when attention has been given to maintaining satisfactory pre-pregnancy circulating concentrations of antiepileptic drugs throughout pregnancy, the impaired seizure control seems to be avoided.
There is little published evidence of maternal injury from epileptic seizures during pregnancy or of significantly increased pregnancy complications such as spontaneous abortion or stillbirth, though neonates born to women taking antiepileptic drugs tend to be a little premature, smaller and of lower birth weight for gestation age than would be expected. Seizures during pregnancy do not seem to be a significant cause of foetal damage.
If epileptic seizures ceased occurring during human pregnancy, as migraine attacks often do, there would be relatively little justification for the existence of the present book. Unfortunately seizures do occur in pregnant women. Therefore certain possibilities need to be considered: (1) Does the physiological state of pregnancy in its own right temporarily or permanently modify the course of seizure disorders? (2) Does the altered disposition of antiepileptic drugs during pregnancy (see Chap. 3) affect the occurrence of seizures? (3) Does having epilepsy, and in particular experiencing epileptic seizures during pregnancy, influence the course of pregnancy or affect the well-being of the foetus?
Effects of Pregnancy on Seizure Disorders
Catamenial Epilepsy
The entity of catamenial epilepsy reflects the fact that, at least in some women, epileptic seizures tend to be experienced at particular stages of the menstrual cycle . The circulating steroidal sex hormone changes that take place during the ovulatory menstrual cycle are a forerunner to those hormonal changes that occur in the earlier weeks of pregnancy. Therefore what happens to seizure occurrence across the menstrual cycle may be seen to provide a possible precedent to the behaviour of seizure disorders during pregnancy.
Depending on how the term ‘catamenial epilepsy ’ is defined, it appears that one-third to one-half of women with epilepsy may have this disorder, in which seizures show a proclivity to occur at mid-cycle or around the time of menstruation. Herkes et al. (1993) used measurements of the course of daily salivary progesterone concentrations throughout the menstrual cycle to define if, and when, catamenial seizures occurred. They studied 20 menstrual cycles (12 ovulatory and 8 anovulatory) in 11 women with catamenial epilepsy who were not using oral contraceptives . In the ovulatory cycles, there were statistically significant increases in seizure frequency at the onset of the rise in progesterone concentrations at mid-cycle and also increases in seizures premenstrually, but in anovulatory cycles, there were only premenstrual increases. Allowing for some possible time lag in hormonal effects, this behaviour of seizure occurrence appears to correlate in a general way with the knowledge that oestrogens increase the excitability of the brain and therefore the chance of having seizures, while progestogens decrease it (Morrell 2002). The decreased cerebral excitability known to be associated with progesterone intake may be mediated mainly by the non-hormonally active progesterone metabolite allopregnanolone which acts at GABAA receptors . However, additional factors may bear on the situation.
It is possible that concurrent antiepileptic drug therapy in women with epilepsy may alter sex hormone concentrations during the menstrual cycle . As mentioned in Chap. 2, the so-called ‘inducing’ antiepileptic drugs not only increase the activities of CYP isoenzymes, including CYP3A4 , but also that of UDP-glucuronosyl transferases which catalyse the metabolism of oestrogens and progestogens (Reddy 2010). Possibly such enzyme induction could cause reduced circulating concentrations of the steroidal sex hormones and by doing this influence the tendency to catamenial patterns of seizure occurrence. We have not traced investigations that have studied this possibility in detail.
Another relevant possibility is that the hormonal changes of the menstrual cycle may alter circulating concentration of antiepileptic drugs in a cyclic pattern and that this alteration may influence the timing of seizure occurrence during the cycle. Some information is available concerning antiepileptic drug clearance changes across the menstrual cycle. In some women there appears to be a tendency for plasma phenytoin levels to fall, relative to drug dose, around the time of menstruation (Rosciszewska et al. 1986). In keeping with this observation, Shavit et al. (1984) showed that the clearance of phenytoin tended to be higher at the time of menstruation than at mid-interval and that the elimination half-life of the drug was a little shorter premenstrually. Wegner et al. (2010) measured plasma lamotrigine concentrations every second day across the menstrual cycle in 7 women who were not taking oral contraceptives and found no statistically significant change in lamotrigine clearance. Also in women who were not using oral contraceptives , Herzog (2009) found a nonstatistically significant 31.3 % decrease in circulating lamotrigine levels and an 8.3 % decrease in valproate ones, in the mid-luteal phase as compared with the mid-follicular phase of the menstrual cycle. Both of these groups of workers also studied women taking oral contraceptives . In these women, clearances of the antiepileptic drugs concerned were statistically significantly increased in the stage of the cycle when synthetic hormone intake was present.
Overall, the above data suggest that during the menstrual cycle , variation in plasma concentration of antiepileptic drugs relative to drug dose and physiological or iatrogenically produced sex hormonal level changes may both contribute to the catamenial occurrence of epileptic seizures. Is this experience consonant with what happens in women with epilepsy during their pregnancies?
Seizure Occurrence Rates in Pregnancy
At first sight, it might seem simple enough to determine whether pregnancy in its own right altered the rate of occurrence of epileptic seizures. In practice, attempts to settle the matter have been confounded by the possible effects of the antiepileptic drug therapy that is usually taken during pregnancy by women with epilepsy.
The first at least modestly effective antiepileptic agents began to come into use after 1857 when Locock’s comments about the effectiveness of potassium bromide in ‘hysterical’ epilepsy appeared in the medical press (Eadie 2012). The effects of these comments were soon afterwards reinforced by Wilks (1861) who, unaware of Locock’s comments, published a report of the drug’s effectiveness in a small series of patients with epilepsy. Over the subsequent century and a half, further agents possessing greater antiepileptic efficacies became available and were then used extensively. As a result, at least in Western societies, for very many years there has been almost no experience of the natural history of untreated epilepsy . Attempts have been made to remedy this deficiency in knowledge of the course of the untreated disorder by studying the natural history of epilepsy in societies where drug treatments are unavailable or are too expensive for widespread use. Unfortunately, in those societies the aetiologies of epilepsy may be different from those in Western societies, and medical records, if available, are less likely to be adequate for later study. If one were to attempt to ascertain the course of untreated, or ineffectively treated, epilepsy in Western societies before the introduction of potassium bromide in the mid-nineteenth century, there would again be problems. Prior to that time, relatively few statistical data concerning epilepsy had been published, and the clinical spectrum of the disorder then diagnosed as ‘epilepsy’ was considerably less extensive than it now is. In the latter half of the nineteenth century, it was rather widely held that epilepsy was a disease in its own right, one with no detectable pathological basis, though Gowers (1881) in his monograph seemed to accept that such epilepsy could also be due to inactive, though not to active, brain pathology. Hence epileptic seizures attributed to a recognised cause were often not included in the epilepsy statistics at the time when no effective therapy was available. Herpin’s posthumous monograph, which described the wealth of epileptic aura and minor seizure phenomena, appeared before 1870 (Herpin 1867), but it received little notice until near the end of the century. Consequently, focal epilepsies which did not culminate in convulsing also tended not to be included in epilepsy statistics until well after potentially effective antiepileptic drug therapy in the form of potassium bromide was available. Therefore the few available statistics concerning seizure frequency that were collected prior to 1850 usually applied to generalised convulsive seizures that had no recognised underlying pathological cause. Such data do not easily equate with what is today considered to be epilepsy.
The above matters and the evidence that plasma levels of many antiepileptic drug levels fall, relative to dose, during pregnancy (Chaps. 3, 4, 5 and 6) need to be kept in mind when considering what is known about the reported behaviour of epileptic seizure disorders during pregnancy.
Nineteenth-century monographs on epilepsy do contain some statements concerning the course of seizure disorders during pregnancy. For the most part, these statements seem to have been based not on quantitative data but on individual authors’ impressions or on memories of particular instances, e.g. of a woman with previously active epilepsy who became seizure-free while pregnant. The account of Raoul Béraud in a University of Paris MD thesis in 1884 is probably the first to contain useful statistics. In his series of 31 pregnancies, seizures became more frequent in 26 % and less frequent in 48 % and were unaltered in frequency in the remaining 26 %. Béraud concluded that (1) pregnancy did not cause epilepsy or modify the pattern of an individual’s seizures and that (2) the influence of pregnancy on epilepsy did not extend beyond the duration of pregnancy. He stated that epilepsy did not predispose to eclampsia and that maternal bromide intake was not harmful to the foetus. This latter item of information suggests that not all of his collection of pregnancies had been untreated.
Knight and Rhind (1975) studied 153 pregnancies in 59 women with epilepsy. Seizure frequency increased during pregnancy in 45.2 % and decreased in 4.8 % and was unchanged in the remainder. A few years later, Schmidt (1982) summarised the numerical data concerning the effects of pregnancy on seizure frequency that could be found in the available literature published during the preceding century. In total, he found record of 2165 pregnancies in women with epilepsy. Seizures had become more frequent in 24.1 % of these pregnancies and less frequent in 22.7 % and were unaltered in frequency in 53.2 %. He recognised that different authors had employed different criteria for determining seizure improvement or worsening. Possibly because of this, he did not pursue his analysis in greater detail. The individual 28 data sets that he analysed contained substantially different numbers of pregnancies. Seizure numbers were in fact increased in pregnancy in 16 of the data sets and decreased in 10. All the series dated from times when antiepileptic drug therapy was available, but it was only at the end of the time period that Schmidt surveyed that the possible implications of altered antiepileptic drug disposition during pregnancy began to be appreciated.
In the following year, Schmidt et al. (1983) reported on their monitoring of the course of 136 pregnancies in 122 women with epilepsy. In 37 %, the number of seizures increased during pregnancy or the puerperium , and in 13 % the number decreased. In half of their pregnancies, the rate of seizure occurrence was unaltered. Otani (1985) found that 27 % of a series of 125 women with epilepsy were poorly compliant with antiepileptic drug therapy during pregnancy. The seizure frequency was unaltered in 80 % of the remaining women, increased in 16 % and decreased in 4 %. Bardy (1987) compared seizure numbers in the pre-pregnancy year, during pregnancy and in the three postnatal months, in 140 women with epilepsy. Seizure numbers increased during pregnancy in 32 % and decreased in 14 %. Gjerde et al. (1988), on the other hand, found no statistically significant difference in seizure occurrence rates before and during pregnancy.
Sabers and Dam (1990) analysed the literature reports of seizure occurrence during pregnancy that had been published between 1884 and 1987. This data set must have largely coincided with that of Schmidt (1982) referred to immediately above. Sabers and Dam subdivided their material into three time periods, viz. 1884–1937, 1938–1970 and 1971–1987. They showed that the proportions of pregnancies in which seizure frequency was unchanged increased from 26 %, through 47 % to 58 % over these periods, that the proportions of pregnancies with increased seizures fell from 42 % through 35 % to 28 % and that the proportions with decreased seizures fell from 32 % through 18 % to 14 %. Thus in all three time periods, seizure frequency increased in pregnancy more often than it decreased. Tanganelli and Regesta (1992) found seizure frequency unchanged in nearly 80 % of pregnancies in their women with epilepsy.
In the past quarter of a century, further case series have been published whose findings are increasingly relevant to the current clinical situation regarding epilepsy. For instance, Chen et al. (2009) remarked on a statistically significant increase in seizures in 1016 pregnancies in Taiwanese women, and Yerby (2008) stated that, in the literature, seizures had increased in one-quarter to one-third of the pregnancies of women with epilepsy. Further, the increase was unrelated to the seizure type that was present.
More recently, there has been an increasing trend to report results of seizure disorder behaviour during pregnancy in terms of whether pregnancy was or was not seizure-free, rather than in terms of alterations in the numbers of seizure that occurred. This all or nothing criterion has become increasingly significant in relation to the life situations of women with epilepsy when loss of seizure control increasingly limits the sufferer’s lifestyle, particularly in relation to vehicle driving. The EURAP Study Group (2006) reported that 58.3 % of 1956 pregnancies in their pregnancy registry had been seizure-free. Mawer et al. (2010) found that 50 % of their series of 277 pregnancies in women with epilepsy were seizure-free throughout pregnancy. Battino et al. (2013) subsequently indicated that 66.6 % of 3806 pregnancies in the EURAP registry that had been treated with carbamazepine , phenobarbitone , valproate or lamotrigine monotherapy had remained seizure-free throughout pregnancy. Women with genetic generalised epilepsies were more likely than women with focal epilepsies to experience seizure-free pregnancies (73.6 % as compared with 59.5 %). Seizure control had worsened during pregnancy in 15.8 % of the EURAP pregnancies. It was noted that the pregnancies managed with lamotrigine monotherapy were less likely to be seizure-free than the remaining treated pregnancies. Reisinger et al. (2013) found that, in 115 pregnancies in 95 women with epilepsy, seizures were more likely to occur during pregnancy if the epilepsy involved was a focal one.
Some Australian Pregnancy Register data are probably included in the above EURAP data, but the amount is unknown. The Australian Register has continued to accumulate pregnancies since the time of the Battino et al. (2013) analysis. Of 1592 antiepileptic drug-treated pregnancies in the Australian Register by the end of 2013, some 53.5 % had been seizure-free throughout pregnancy. In 692 of these 1592 Australian pregnancies, the women involved had suffered seizures in their pre-pregnancy year, whereas in the remaining 900 the women (56.5 %) had been free from seizures for at least a year before becoming pregnant. Of those with active seizure disorders in the pre-pregnancy year, 78.9 % continued to have seizures during pregnancy. Of the 900 who were seizure-free for at least a year before pregnancy, only 21.7 % experienced seizures during pregnancy. Generalised convulsive seizures are more likely to be remembered than more minor epileptic manifestations. In the Australian Register , 258 of the 1592 pregnancies were associated with generalised convulsive seizures in the pre-pregnancy year, but 298 had such seizures during the shorter nine-month duration of pregnancy (16.2 % versus 18.7 %).
Of the pregnancies in the Australian Register , 785 had occurred in women with focal epilepsies and 669 in women with genetic generalised epilepsies (the seizure disorder type being uncertain in the remainder). Seizures of some type had occurred in the pre-pregnancy year in 49.9 % of the pregnancies in women with focal epilepsies and in 53.6 % during pregnancy. In these same women, generalised convulsive seizures had occurred in 28.2 % during the pre-pregnancy year but in 17.1 % during the shorter nine-month period of pregnancy. As to the pregnancies of women with genetic generalised epilepsies , the rate of occurrence of any type of seizure was 37.1 % during the pre-pregnancy year and 39.6 % during pregnancy. Generalised convulsive seizures had occurred in 18.4 % of the pregnancies in this idiopathic/genetic generalised epilepsy subset during the year prior to pregnancy and in 19.9 % during the nine months of pregnancy. Thus, in a series of pregnancies managed by what one would hope, but could not guarantee, was contemporary good therapeutic practice, there still appeared to be a tendency for decreased complete seizure disorder control during antiepileptic drug-treated pregnancy. Nevertheless, more than half of all pregnancies remained totally seizure-free throughout.
Factors Influencing Seizure Occurrence During Pregnancy
The above data suggest that the types of seizure disorder present in a very considerable majority of treated women probably were not significant factors in determining whether or not seizure control deteriorated during pregnancy. In contrast, the overall activity of the seizure disorders in the year before pregnancy appeared to have a significant effect on seizure freedom rates during pregnancy.
In the Australian Register data then available, Vajda et al. (2008) analysed the likelihood of seizures occurring during pregnancy in relation to the duration of seizure freedom prior to pregnancy. The outcome is summarised in Fig. 7.1, which contains data subsequent to the 2008 publication. There appeared to be a substantially lower risk of seizures recurring during pregnancy if there had been 18 months seizure freedom before pregnancy. Longer periods of seizure freedom than this offered almost negligible further advantages. As a general guide, 1 year seizure freedom before pregnancy probably would offer the best compromise between the disadvantage arising from experiencing seizures in pregnancy and the disadvantages from delaying pregnancy for extended periods to minimise the risk of seizures occurring during pregnancy. In the year after the publication of Vajda et al. (2008), Harden et al. (2009) reached a basically similar conclusion but in relation to a 9-month seizure-free interval before pregnancy. Thomas et al. (2012), in a study of 1297 pregnancies, commented that seizures in the pre-pregnancy month increased the risk of further seizures occurring during pregnancy.
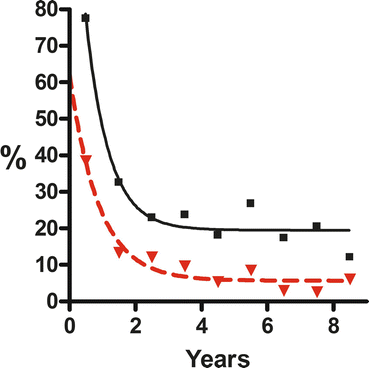

Fig. 7.1
Rates of occurrence during pregnancy for any seizures (continuous line) and for generalised convulsive seizures (broken line), related to duration of seizure freedom prior to pregnancy
Cangetti et al. (2014) drew attention to a further factor of prognostic relevance. They obtained evidence that having had a past history of seizures predominantly in the peri-menstrual few days, or in addition at the approximate time of ovulation , increased the chances of a woman experiencing a pregnancy without seizures or with reduced numbers of seizures.
From the recent literature, it appears that a majority of women with antiepileptic drug-treated epilepsy will remain seizure-free throughout their pregnancies if contemporary optimal therapeutic management practices are followed. Unfortunately, a tendency exists for some women’s apparently fully controlled seizure disorders to relapse during pregnancy or for their seizures to become more frequent. There are several possible explanations for this seizure disorder worsening. Despite existing knowledge of the increased eliminations of many antiepileptic drugs during pregnancy, it is possible that antiepileptic drug dosages have not always been adjusted appropriately during pregnancy to counter the effects of falling circulating drug concentrations. This fall may have been responsible for seizures continuing to occur in 47 % of the pregnant women in the series of Schmidt et al. (1983), as at the time of that study, the importance of maintaining pre-pregnancy plasma concentration of antiepileptic drugs during pregnancy was not as widely appreciated as it now is. Accidental or deliberate noncompliance with recommended antiepileptic drug dosages is another possibility. There is some published evidence, as well as anecdotal data, indicating that noncompliance with prescribed antiepileptic drug therapy does sometimes occur during pregnancy in women with epilepsy (Schmidt et al. 1983). The latter authors also suggested that sleep deprivation in pregnancy could impair seizure control. There is the further possibility that in some women impaired seizure disorder control in pregnancy may be due to the state of being pregnant itself, by altering the intrinsic activity, or the nature, of the underlying epileptogenic process.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


