Table 34-1. Aminoglycosidesa
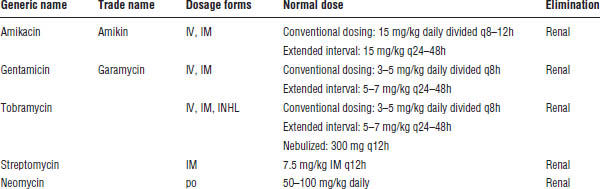
INHL, inhaled; IV, intravenous.
a. Use ideal or adjusted body weight for all aminoglycoside dosing.
34-4. Penicillins
Mechanism of Action
Penicillin-binding proteins make up the bacterial cell wall. When penicillin binds to these proteins, it is able to inhibit cell wall synthesis in the bacteria, causing cell wall lysis and ultimately cell death.
Penicillins are bactericidal; they inhibit bacterial cell wall synthesis. They are known as β-lactam antibiotics because their chemical structure consists of a β-lactam ring adjoined to a thiazolidine ring.
Spectrum of Activity and Dosing
See Tables 34-2 and 34-3 for information about the spectrum of activity and dosing of penicillins, respectively.
Adverse Drug Events
Allergic or hypersensitivity reaction occurs in 3–10% of patients. Rash (4–8% of patients) or anaphylaxis (0.01–0.05% of patients) can occur within 10–20 minutes and is more common in intravenous (IV) than in oral administration.
Neurologic reactions (seizures) are seen with high doses of penicillin given to patients with renal insufficiency.
Gastrointestinal (GI) effects, including nausea and vomiting, may occur with oral use.
Hypokalemia and hypernatremia may occur, particularly with carboxypenicillins.
Penicillinase-resistant penicillins can cause interstitial nephritis and increase transaminases.
Hematologic reactions (thrombocytopenia, neutropenia, hemolytic anemia) are possible.
Drug–Drug Interactions
Probenecid competitively inhibits tubular secretion of penicillins, thus increasing plasma levels. This interaction is sometimes employed in serious central nervous system (CNS) infections to increase drug concentrations.
Concomitant use with an oral contraceptive may decrease the effectiveness of the oral contraceptive and increase incidence of breakthrough bleeding.
Other Characteristics
Nafcillin and oxacillin are eliminated primarily by biliary excretion; therefore, there is no need to adjust dosage for patients with renal dysfunction.
Penicillin G benzathine is a repository drug formulation. When it is given IM, insoluble salt allows slow drug absorption from the injection site, and therefore, it has a longer duration of action (1–4 weeks, dose-dependent).
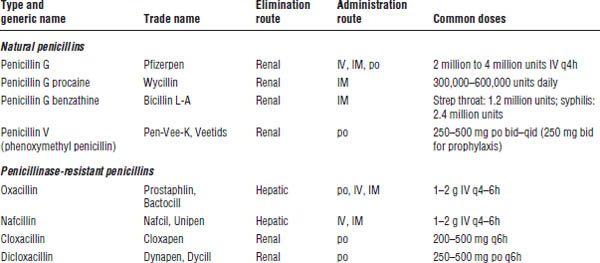
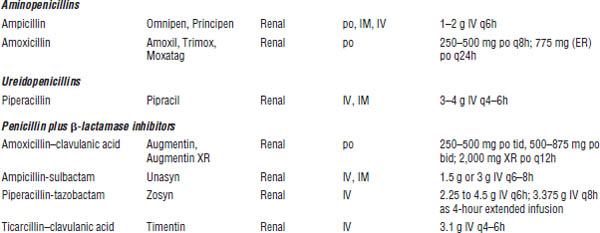
Table 34-2. Spectrum of Activity of the Penicillins
Category | Spectrum |
Natural penicillins | Natural penicillins are effective against viridans group streptococci and Streptococcus pyogenes and against some S. pneumoniae, mouth anaerobes, and Clostridium perfringens (gas gangrene). |
| They are ineffective against Staphylococcus aureus because they are readily hydrolyzed by penicillinases. |
Penicillinase-resistant penicillins | These agents are drugs of choice for methicillin-susceptible Staphylococcus aureus (MSSA). They cover some streptococci but not enterococci. |
Aminopenicillins | Aminopenicillins are drugs of choice for enterococci and Listeria monocytogenes. |
| They cover some Streptococcus pneumoniae, Haemophilus influenzae, Escherichia coli, Klebsiella, and Proteus. |
Carboxypenicillins and | The spectrum is like that of aminopenicillins, but these drugs provide more Gram-negative coverage. They cover Proteus, E. coli, Klebsiella (not ticarcillin alone), Enterobacter, and Pseudomonas. They have in vitro activity against staphylococci, streptococci, enterococci, most Enterobacteriaceae, Pseudomonas, and many anaerobes, including Bacteroides fragilis. Ureidopenicillins possess better in vitro activity against Pseudomonas species than carboxypenicillins (piperacillin > ticarcillin). |
β-lactamase inhibitors | β-lactamase inhibitors are combined with penicillins to improve both Gram-negative and anaerobic activity including enhanced coverage of H. influenzae, Moraxella catarrhalis, Bacteroides, E. coli, and other Enterobacteriaceae. |
Ampicillin-sulbactam and amoxicillin–clavulanic acid | This combination is active against H. influenzae, M. catarrhalis, Proteus, E. coli, Klebsiella pneumoniae, MSSA, and anaerobes. |
Ticarcillin–clavulanic acid | This combination has activity against Pseudomonas aeruginosa, H. influenzae, M. catarrhalis, Proteus, E. coli, K. pneumoniae, MSSA, and anaerobes. It has enhanced activity against the nosocomial pathogen Stenotrophomonas maltophilia in comparison with piperacillin-tazobactam. |
Piperacillin- | This combination provides overall enhanced Gram-positive, Gram-negative (most notably P. aeruginosa), and anaerobic coverage compared with ticarcillin–clavulanic acid. |
34-5. Cephalosporins
Cephalosporins are β-lactam antibiotics that are structurally and pharmacologically similar to penicillins.
Mechanism of Action
Cephalosporins bind to penicillin-binding proteins in a manner similar to that of other β-lactams, thereby inhibiting peptidoglycan synthesis. Cephalosporins are bactericidal agents.
Spectrum of Activity
Cephalosporins are broad-spectrum antimicrobial agents; however, the spectrum of activity varies greatly among the individual agents. Thus, cephalosporins are grouped into four broad classes, or generations, according to their antimicrobial coverage (Table 34-4). It is important to note that no cephalosporin has clinically dependable coverage of enterococci.
First-generation agents (cefadroxil, cefazolin, cephalexin)
Gram-positive activity is extensive, including agents of choice for methicillin-susceptible Staphylococcus aureus (MSSA), and coverage of Streptococcus pyogenes (group A beta-hemolytic streptococci), Streptococcus agalactiae (group B streptococci), and Streptococcus pneumoniae. First-generation agents are inactive against methicillin-resistant staphylococci (methicillin-resistant Staphylococcus aureus [MRSA], methicillin-resistant Staphylococcus epidermidis [MRSE]), and Listeria monocytogenes.
Gram-negative activity is limited, although some strains of Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Shigella may display susceptibility. First-generation agents are inactive against Haemophilus influenzae, Neisseria, Pseudomonas, Enterobacter, Citrobacter, Serratia, other Proteus spp., and anaerobes such as Bacteroides fragilis.
Table 34-3. Dosing of Penicillins
Second-generation agents (cefaclor, cefotetan, cefoxitin, cefprozil, cefuroxime) and cephamycins (cefoxitin, cefotetan)
Gram-positive activity is similar to that of first-generation agents.
Gram-negative activity of second-generation agents is generally more extensive than that of first-generation agents, including some strains of E. coli, Klebsiella, and Proteus. Second-generation agents are active against H. influenzae, Neisseria, and some (cefotetan, cefoxitin) also have anaerobic activity. Second-generation agents are inactive against Pseudomonas.
Third-generation agents (cefdinir, cefixime, cefotaxime, cefpodoxime, ceftazidime, ceftibuten, ceftriaxone)
In general, Gram-positive activity is reduced versus first- and second-generation agents; however, ceftriaxone displays effective S. pneumoniae coverage.
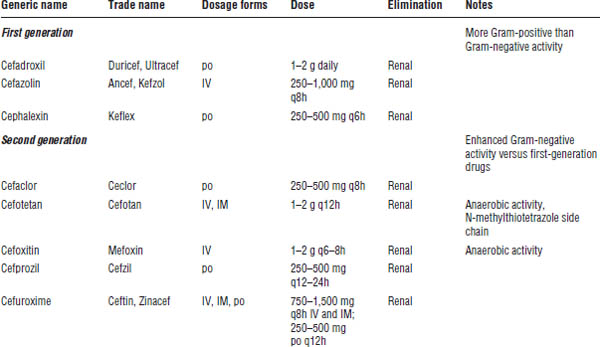
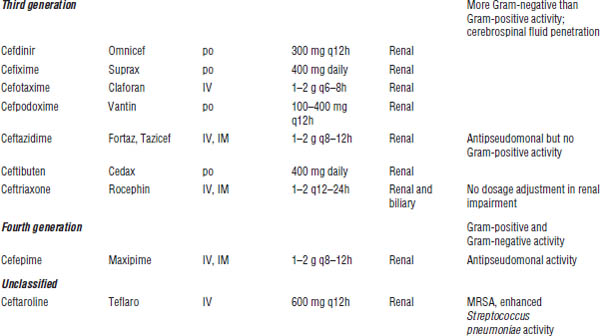
Gram-negative activity is extensive, including Enterobacter, Citrobacter, Serratia, Neisseria, and Haemophilus. Some third-generation agents are active against Pseudomonas (ceftazidime).
Fourth-generation agent (cefepime)
Gram-positive activity is enhanced versus third-generation agents. Cefepime is inactive against MRSA, Listeria, and anaerobes.
Gram-negative activity is extensive, including enhanced activity against Pseudomonas and Enterobacteriaceae that produce inducible β-lactamases.
Unclassified agent (ceftaroline)
The spectrum of activity of ceftaroline is similar to that of the third-generation agent ceftriaxone, except ceftaroline is active against MRSA and has further enhanced S. pneumoniae coverage. Currently, ceftaroline is approved for the treatment of community-acquired pneumonia and skin and soft tissue infections. Ceftaroline is renally eliminated and must be dose adjusted in renal dysfunction.
Adverse Drug Events
■ Hypersensitivity, including fever, rash, pruritus, urticaria, anaphylaxis, and hemolytic anemia
■ GI effects, such as nausea, vomiting, and diarrhea
■ Nephrotoxicity (rare)
■ Seizures (potential risk with high doses in patients with renal impairment)
■ Clostridium difficile colitis
■ Bleeding or hypoprothrombinemia (cefotetan), which is attributable to the presence of an N-methylthiotetrazole (NMTT) side chain in the structure of these agents (possible prevention or reversal with administration of vitamin K)
■ Blood dyscrasias (rare)
Drug–Drug Interactions
Disulfiram-like reactions have been reported with ingestion of alcohol during treatment with cephalosporin antibiotics that possess an NMTT side chain (cefotetan).
Probenecid competitively inhibits tubular secretion of cephalosporins, resulting in higher serum concentrations.
Drug–Disease Interactions
All cephalosporins (except ceftriaxone) require dosage adjustments in patients with renal insufficiency.
Monitoring Parameters
Serum concentration monitoring is not necessary. Patients should be monitored for clinical response and resolution of infection.
Patient Instructions and Counseling
Verify that the patient is not allergic to penicillins. Traditional teaching principles have noted that cross-sensitivity with penicillins has been reported in up to 10% of patients receiving cephalosporins; however, recent evidence suggests that overall cross-reactivity may be much lower (around 1–2%) and even less likely with third- and fourth-generation cephalosporins (less than 1%). Obtain a thorough history of any patient with a previous allergic reaction to a β-lactam antibiotic. Cephalosporins should be avoided in patients with a severe hypersensitivity reaction (anaphylaxis) to penicillins.
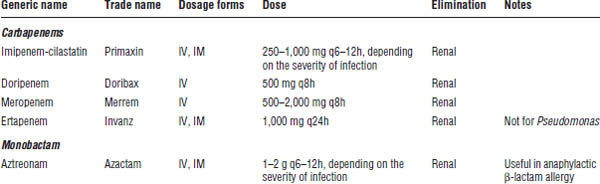
34-6. Carbapenems
Carbapenems are β-lactam-like antibiotics that are structurally and pharmacologically similar to penicillins (Table 34-5). Currently, only IV dosage forms are available for carbapenems.
Mechanism of Action
Carbapenems bind to penicillin-binding proteins in a manner similar to that of other β-lactams, thereby inhibiting peptidoglycan synthesis. Carbapenems are bactericidal in susceptible isolates.
Spectrum of Activity
Carbapenems are very broad-spectrum antibiotics with activity against most Gram-positive and Gram-negative aerobes and anaerobes, as well as activity against some Mycobacterium spp. They are the drugs of choice for ESBL (extended spectrum β-lactamases)–producing Enterobacteriaceae species. Carbapenems other than ertapenem cover Pseudomonas and some Acinetobacter spp.
Adverse Drug Events
GI adverse effects are the most common events reported with imipenem. The effects include nausea, vomiting, diarrhea (including C. difficile enterocolitis), gastroenteritis, and abdominal pain.
Table 34-5. Carbapenems and Monobactam
Eosinophilia, leukopenia, neutropenia, agranulocytosis, hemolytic anemia, and thrombocytopenia have been reported.
Seizures have been reported in approximately 0.4% of patients receiving imipenem. Carbapenems have the highest seizure-risk profile of the β-lactams, and imipenem likely poses the greatest risk of the carbapenem class. Risk factors include the following:
■ History of seizures or head trauma
■ High doses
■ Renal dysfunction
Imipenem-Cilastatin
Imipenem is a semisynthetic carbapenem β-lactam antibiotic. Cilastatin prevents renal metabolism of imipenem by dehydropeptidase—an enzyme present on the brush border of the proximal renal tubule—thereby increasing the concentrations of the active drug and preventing production of a nephrotoxic metabolite. Cilastatin has no antibacterial activity.
Meropenem and Doripenem
These agents are similar to imipenem with the following differences:
■ Slightly lower seizure risk
■ No hydrolysis by dehydropeptidases
Ertapenem
Ertapenem is dosed once daily and does not cover Pseudomonas spp.
34-7. Monobactam
Monobactam antibiotics are cell wall–active antibiotics like the β-lactams, but slight structural differences make them weakly immunogenic, decreasing allergic cross-reactivity with penicillins and cephalosporins (Table 34-5).
Aztreonam
Aztreonam is the only monobactam antibiotic currently available.
Mechanism of action
Monobactams bind to penicillin-binding proteins in a manner similar to that of other β-lactams, thereby inhibiting peptidoglycan synthesis. Monobactams are bactericidal in susceptible isolates.
Spectrum of activity
Aztreonam is active against many aerobic Gram-negative bacteria, but is not active against any Gram-positive or anaerobic bacteria. Though some strains of Pseudomonas are susceptible, resistance is increasing.
Adverse drug events
■ Hypersensitivity: Rash, injection site reactions, eosinophilia
■ GI effects: Nausea, vomiting, diarrhea
34-8. Gram-Positive Antibiotics
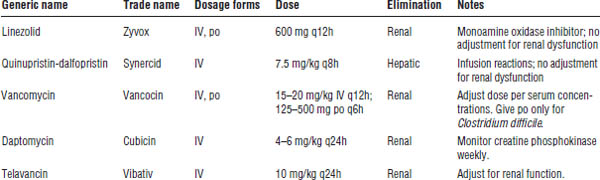
Linezolid
Linezolid is a synthetic oxazolidinone antibiotic (Table 34-6).
Mechanism of action
Linezolid binds to the 23S site of the 50S ribosomal subunit that inhibits bacterial translation and thus protein synthesis.
Spectrum of activity
Linezolid is active against Staphylococcus spp., including MRSA; Enterococcus faecalis and E. faecium isolates, including vancomycin-resistant enterococci (VRE); and Streptococcus spp., including S. pneumoniae. Linezolid is primarily classified as a bacteriostatic antibiotic.
Adverse drug events
Hematologic effects, including myelosuppression (primarily as thrombocytopenia, but also anemia, leukopenia, pancytopenia), have been reported. Hematologic effects are more common when therapy exceeds 14 days and appear to be reversible on discontinuation of the agent. Neurotoxicity such as peripheral neuropathy and optic neuritis has been reported with prolonged use of linezolid.
Linezolid is a weak monoamine oxidase inhibitor (MAOI), and caution should be exercised in patients receiving other serotonergic agents (MAOIs, SSRIs [selective serotonin reuptake inhibitors], TCAs [tricyclic antidepressants], meperidine, triptans) or sympathomimetics (pseudoephedrine, norepinephrine, dopaminergic agents). Serotonin syndrome has been reported, primarily in patients receiving multiple agents with serotonergic activity.
Other
Linezolid is a bacteriostatic agent that achieves higher concentrations in tissue than in plasma. Clinical data recommend against its use in bacteremia because of increased mortality.
Quinupristin-Dalfopristin
Quinupristin-dalfopristin is a semisynthetic streptogramin antibiotic. The combination acts synergistically against Gram-positive bacteria.
Mechanism of action
Quinupristin inhibits late-phase protein synthesis, while dalfopristin inhibits early-phase protein synthesis through binding to the 50S subunit of bacterial RNA.
Spectrum of activity
Quinupristin-dalfopristin is bactericidal against staphylococci (including MRSA) and streptococci and bacteriostatic against E. faecium, including VRE. Quinupristin-dalfopristin is not active against E. faecalis.
Table 34-6. Gram-Positive Antibiotics
Adverse drug events
Tolerability and adverse effects often preclude the use of this agent. Thrombophlebitis and severe injection site reactions are common, and the drug should be administered through a central venous catheter only.
Hyperbilirubinemia has been reported in up to 25% of patients receiving the agent.
Arthralgias and myalgias are common, some requiring discontinuation of the agent.
Other
The U.S. Food and Drug Administration (FDA) has recently repealed the VRE treatment indication for quinupristin-dalfopristin, citing the inability to verify clinical benefit.
Daptomycin
Daptomycin is a cyclic lipopeptide antibiotic.
Mechanism of action
Daptomycin binds to bacterial cell membranes, causing rapid depolarization, which results in loss of membrane potential. The loss of membrane potential inhibits protein, deoxyribonucleic acid (DNA), and RNA synthesis, resulting in cell death.
Spectrum of activity
Daptomycin is bactericidal against the Gram-positive bacteria staphylococci (including MRSA), streptococci, and enterococci (including VRE).
Adverse drug events
Dermatologic reactions include injection site reaction, rash, and pruritis. Musculoskeletal effects include increased creatine phosphokinase (CPK), which can progress to rhabdomyolysis (weekly monitoring recommended). Increased caution and CPK monitoring should be performed with concomitant use of daptomycin and statin therapy. Rarely, pulmonary eosinophilia manifesting as eosinophilic pneumonia has been reported.
Other
Daptomycin is inactivated by the surfactant in the lung and cannot be used to treat pneumonia.
Glycopeptide Antibiotics
Mechanism of action
The glycopeptides vancomycin and telavancin exhibit bactericidal killing through inhibition of peptidoglycan synthesis polymerization and cross-linking, and thus cell wall synthesis. This binding occurs at a site different from that of the penicillins.
Vancomycin
Spectrum of activity
Vancomycin is active against most Gram-positive bacteria, such as staphylococci (including MRSA), streptococci, and enterococci. It is bactericidal and acts synergistically with aminoglycosides for Gram-positive pathogens. Vancomycin is active against C. difficile and can be used to treat C. difficile infection when administered in oral dosage form.
Adverse drug events
Nephrotoxicity is manifested by an increase in serum creatinine and BUN. The incidence of nephrotoxicity is controversial but appears to be higher with concomitant nephrotoxic agents, high trough concentrations, and higher total daily doses. Renal dysfunction is normally reversible on discontinuation of the agent but may be irreversible.
Ototoxicity is induced by eighth cranial nerve damage and has been reported to cause permanent hearing loss. Vancomycin rarely causes vestibular toxicity. The incidence of ototoxicity appears to be low in the absence of concomitant ototoxic agents.
Thrombophlebitis is common and requires frequent IV site rotation.
Histamine release, or “red-man syndrome,” is a reaction most commonly associated with rapid IV infusion. Histamine reactions can be minimized by slow IV infusion, not to exceed 500 mg/30 min and through the use of antihistamines such as diphenhydramine (Benadryl).
Monitoring parameters
Vancomycin trough concentrations should be monitored, whereas monitoring of vancomycin peak concentrations is not routinely required.
Pharmacokinetics
Vancomycin is renally eliminated:
■ t1/2 = 6 hours (normal renal function)
■ t1/2 = 7–10 days (anephric patients)
■ Vd = 0.7 L/kg (TBW)
■ Dose = 15–20 mg/kg (TBW) (Loading dose of 25–30 mg/kg may be considered.)
■ Interval = q8h to intermittent dosing (based on renal function and pharmacokinetic monitoring)
■ Peak concentration = 20–40 mcg/mL
■ Trough concentration = 10–20 mcg/mL for pathogens with an MIC of ≤ 1 mg/dL. For serious infections (bacteremia, endocarditis, osteomyelitis, meningitis, pneumonia), trough concentrations of 15–20 mcg/mL are recommended. Lower trough concentrations of 10–15 mcg/mL are reserved for skin and soft tissue infections. An alternative Gram-positive agent should be used with an MIC ≥ 2 mg/dL to vancomycin.
Telavancin
Telavancin is a bactericidal lipoglycopeptide antibiotic that inhibits cell wall synthesis. Telavancin is available only intravenously and is currently indicated for complicated skin and soft tissue infections.
Spectrum of activity
Telavancin is active against Gram-positive bacteria such as staphylococci (including MRSA), streptococci, and vancomycin-susceptible enterococci.
Telavancin is renally eliminated, and dose and interval may be adjusted for renal dysfunction. Clinical trials have shown that efficacy may decrease with increasing age or renal dysfunction. Currently, no drug monitoring is recommended or available for telavancin.
Adverse drug events
Injection site reactions are common, including pain, pruritis, erythema, and rigor.
Nephrotoxicity is reported more often with telavancin than with vancomycin.
Neurologic effects such as dizziness, headache, and insomnia are also common.
QT prolongation can occur.
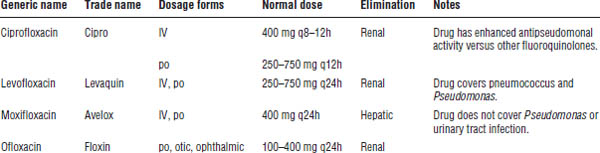
34-9. Fluoroquinolones
Quinolones are broad-spectrum antibacterial agents (Table 34-7).
Mechanism of Action
Fluoroquinolones are bactericidal agents. The mechanism of action of these agents is inhibition of topoisomerase II (DNA gyrase) and topoisomerase IV, resulting in disruption of bacterial DNA replication.
Spectrum of Activity
Gram-negative activity is extensive, including E. coli, Klebsiella, Proteus, Enterobacter, Citrobacter, Salmonella, and Shigella, in addition to Moraxella catarrhalis and H. influenzae. Activity against Pseudomonas aeruginosa varies among individual agents.
Newer fluoroquinolones (levofloxacin, moxifloxacin) demonstrate superior Gram-positive coverage versus older agents (ciprofloxacin, ofloxacin). Fluoroquinolones have limited enterococcal activity and are not recommended for the treatment of invasive staphylococcal infections or MRSA.
Moxifloxacin has some anaerobic coverage and can be used as an alternative treatment for aspiration pneumonia.
All fluoroquinolones are highly active against Legionella and display atypical coverage.
Adverse Drug Events
■ GI effects: Nausea and dyspepsia
■ CNS effects: Peripheral neuropathy, headache, dizziness, and insomnia
■ Cardiovascular effects: QT prolongation (moxifloxacin > levofloxacin and ciprofloxacin). Avoid use in patients with preexisting QT prolongation.
■ Endocrine effects: Hypoglycemia or hyperglycemia (reason for FDA withdrawal of gatifloxacin)
■ Other effects: Tendinitis and tendon rupture (highest risk patients > 60 years of age, concomitant use of corticosteroids, transplant patients)
■ Rare effects: Rash, urticaria, leukopenia, and hepatotoxicity (reason for FDA withdrawal of trovafloxacin)
Drug–Drug Interactions
Ciprofloxacin increases theophylline levels. Concomitant use should be avoided, or theophylline levels should be monitored during treatment. The risk of theophylline toxicity is lower with other fluoroquinolones.
Antacids, sucralfate, and divalent or trivalent cations (calcium, magnesium, iron) significantly decrease the absorption of fluoroquinolones. These agents should not be administered for at least 2 hours after each dose of a fluoroquinolone.
Fluoroquinolones may enhance the effects of oral anticoagulants. Monitor prothrombin time (PT) and international normalized ratio (INR) if concomitant therapy cannot be avoided.
Concomitant use of fluoroquinolones with agents that prolong the QT interval, particularly moxifloxacin, should be avoided when possible.
Drug–Disease Interactions
Dosage adjustments should be made for renally cleared fluoroquinolones based on specified CrCl except for moxifloxacin. Avoid use in myasthenia gravis.
Monitoring Parameters
Serum concentrations are not monitored. The patient should be monitored for clinical response and resolution of infection.
Patient Instructions and Counseling
■ Fluoroquinolones should be avoided in children or pregnant or nursing females because of the risk of cartilage erosion in tendons and growing bone tissue.
■ Do not take antacids; multivitamins; or other calcium, magnesium, or iron supplements for at least 2 hours after each dose.
34-10. Macrolides
Mechanism of Action
Macrolides (and ketolides) are bacteriostatic against susceptible organisms (Table 34-8). The agents bind to the 50S ribosomal subunit, thereby inhibiting RNA synthesis.
Table 34-8. Macrolides and Ketolides
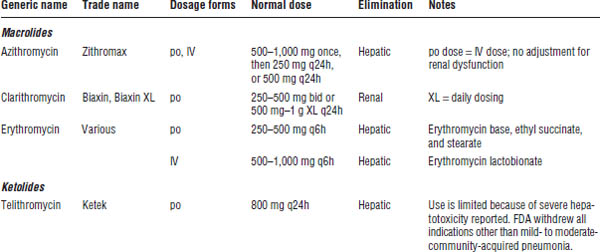
Spectrum of Activity
Macrolides are active against some Gram-positive organisms, including penicillin-resistant streptococci. The macrolides are also effective against Chlamydia, Mycoplasma, Ureaplasma, spirochetes, and mycobacteria.
Macrolides are the drugs of choice in atypical pneumonia and Chlamydia sexually transmitted diseases. Erythromycin is sometimes used to accelerate gastric emptying because of its stimulation of GI motility.
Adverse Drug Events
■ GI effects: Erythromycin stimulates GI motility, leading to abdominal pain and cramping, nausea, vomiting, and diarrhea. Clarithromycin appears to be the least stimulating to the GI tract.
■ Local effects: Erythromycin lactobionate causes venous irritation and thrombophlebitis. The agent should be diluted in at least 250 mL and infused over 30–60 minutes to decrease the venous irritation. It is not commonly used in this dosage form because of these factors.
■ Cardiac effects: QT interval prolongation and arrhythmias have been reported. The risk of arrhythmia is highest in patients with known QT prolongation, hypokalemia, hypomagnesemia, bradycardia, and concomitant use of other antiarrhythmic agents.
■ Hepatotoxicity: Telithromycin carries a black box warning for severe hepatotoxicity.
34-11. Tetracyclines and Glycylcyclines
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


