Anterior and Posterior Colporrhaphy
Dionysios K. Veronikis
Cystocele
The biggest imitator of anterior complex, combined vaginal segment defects is a cystocele. Genital prolapse and especially the anterior vaginal segment remains one of the most challenging aspects of reconstructive pelvic surgery. Anterior segment prolapse, the cystocele, is due to inadequate support and/or insufficient stabilization at rest or during increases in intra-abdominal pressure. Paravaginal defects refer to disturbances in the attachment of the anterior sulcus of the vagina to a connective tissue “bridge” attaching the anterior sulcus to the arcus tendineus fascia pelvis. The paravaginal defect clinically presents as a cystocele due to a lateral separation of the vaginal wall attachments from the lateral pelvic sidewall(s). According to Bayden and Walker, such a detachment should be suspected when the anterior vaginal wall is relaxed. If there is no elevation of the anterior vaginal wall when the patient squeezes the pelvic musculature, the connective tissue and vascular supports of the anterior vaginal wall may have been detached from the arcus tendineus fascia pelvis. The lateral vaginal wall is not attached directly to the arcus tendineus fascia pelvis; instead, the anterior vaginal sulcus is attached to the arcus tendineus fascia pelvis by a meshwork of intervening connective tissue—a connective tissue “bridge.” This intervening connective tissue may be subject to various strains and stretching and partial or complete avulsion. This type of strain is more common in parous patients as a consequence of trauma during labor and delivery and perhaps can also be attenuated, stretched, or avulsed with pull of massive genital prolapse, even in the nulliparous woman.
In 1905, Ernest W. Hey Groves published a paper entitled “bridge.” He stated in this manuscript that “If we regard the cystocele as a hernia, whose radical cure we have to undertake, it is not surprising that methods which depend for their success upon the fixation of so distensible a structure as the vaginal wall should lead to failure.” He explained that “vaginal cystocele exists under two conditions, that in which it only forms part of a uterine descent, and that in which it forms a distinct hernia or pouch, pushing the vagina before it, but leaving the uterus in a normal position.” He further described the levator ani muscle complex, the “bridge” of the pelvis fascia, and its insertion. Although the description of his operative repair is not completely clear, he described the following: “A transverse incision is made from one labium majus to the other, about 3 cm behind the urethral orifice. The incision divides the whole thickness of the vagina. . . . The margins of the levatores ani muscles are then sought for and defined in either angle of the wound. It will be found that they can easily be brought into apposition with little or no tension.”
The first description of lateral cystocele repair incorporating the arcus tendineus fascia pelvis into the cystocele repair was by George R. White in 1909. White performed this repair on 19 patients over a 3-year period, as he stated always in connection with some other plastic operation.
White’s classic paper described a surgical procedure that was designed to correct cystocele—a radical cure—by attaching the lateral vaginal sulci to the arcus tendineus fascia pelvis. One has to wonder what is meant by the term “bridge” because it could conceivably include as much a displacement cystocele from lateral detachment as a displacement cystocele from an apical prolapse. White’s 1909 paper did not receive much attention, and he again reported his views in 1912. Interestingly, based on cadaver dissections, White wrote, “The easiest and simplest way to accomplish this is to incise the peritoneum at the side of the bladder, push the bladder aside until the white line comes into view, and then by the aid of an assistant’s finger in the vagina, suture the anterior lateral side of the vagina to the white line of the pelvic fascia.” Almost 50 years later, in 1961, John Christopher Burch, in performing a Marshall–Marchetti–Krantz operation, was unable to secure the sutures to the periosteum and concomitantly noticed the intravaginal finger elevating the vaginal wall to the “bridge”—the arcus tendineus fascia pelvis. After seven successful procedures he felt that the Cooper ligament was a stronger attachment point. The paravaginal defect and its repair as we know it today were redescribed by Richardson through an abdominal retropubic exposure instead of the original transvaginal approach used by White.
Cystocele and the Paravaginal Defect
Cystocele is a consequence of trauma/damage to the vagina, to the apical vaginal supports, to the lateral vaginal attachments, or to the vaginal wall itself. In examining and evaluating the anterior vaginal wall one must have in mind that there are several distinct types of cystoceles and each may contribute partially or totally to anterior segment defects. All of the existing combinations must be recognized and corrected if surgical treatment is to be effective and durable.
Cystocele has been described according to whether it is anterior to the interureteric ridge (anterior cystocele) or posterior to it (posterior cystocele). Anterior cystocele is synonymous with urethrocele, in which straining or Valsalva efforts result in downward bulging or rotational descent of the urethra with or without a demonstrable tendency of the bladder to “bridge” behind the vesicourethral junction. The distal third of the vaginal wall and attachments underlie and support the urethra. This rotational descent of the vesicourethral junction may be of varying degrees and is often associated with urinary incontinence. Pathologic dilation of the midurethra and/or distal urethra (true urethrocele) is quite uncommon. A true cystocele therefore does not include the distal vagina. Similarly, in patients with an intact cervix and uterus, the cervix occupies the upper third or so of the anterior vaginal wall. The length of the anterior vaginal wall plus the length of the cervix roughly equals the length of the posterior vaginal wall. Thus, a distention cystocele is between the bladder neck and cervix. A distention cystocele (Fig. 1) is almost without exception the result of an overstretching of the vaginal wall beyond its ability to recover/involute after vaginal delivery, or the consequence of the atrophic changes of aging upon the intrinsic structural components of the vaginal wall with or without earlier damage. Rugal folds once visible on the anterior vaginal epithelium diminish with aging and may disappear as the physiologic/pathologic changes behind the squamous epithelium and within the vaginal wall deep to the squamous epithelium progress. These processes allow the anterior vagina and bladder to “bridge” into
the vaginal lumen. In a true distention cystocele the lateral attachments of the vagina are well supported. The transverse topographic length from vaginal sidewall to vaginal sidewall is increased. Therefore, almost without exception the true distention cystocele is the result of attenuation of the anterior vaginal wall. The vagina as an organ responds to physiologic forces as well as pathologically with the same response, that is, by stretching.
the vaginal lumen. In a true distention cystocele the lateral attachments of the vagina are well supported. The transverse topographic length from vaginal sidewall to vaginal sidewall is increased. Therefore, almost without exception the true distention cystocele is the result of attenuation of the anterior vaginal wall. The vagina as an organ responds to physiologic forces as well as pathologically with the same response, that is, by stretching.
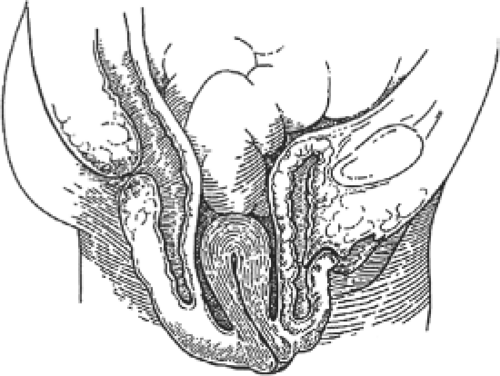
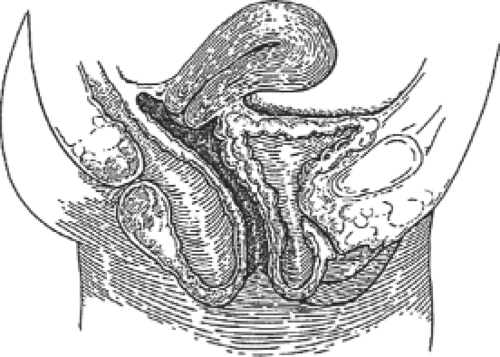 Fig. 1. Sagittal section showing distention cystocele and rectocele. Note that the vaginal vault and uterus are at a normal position within the pelvis. |
Lateral displacement cystocele (Fig. 2) is the result of pathologic elongation or detachment of the lateral vaginal attachments and support of the vagina. Etiologically, this is quite different from distention cystocele caused by connective tissue damage to the normal vaginal wall. The anterior vaginal segment also has superior attachments to the cervix. Thus, the tissues supporting both the vagina and cervix are component parts of the cardinal ligaments and function as an anatomic unit. Displacement of one component is often followed by similar displacement of the other. Posthysterectomy eversion or prolapse of the upper vagina may also present as displacement cystocele by creating a “bridge.” If the upper vaginal segment does not descend, there cannot be a telescoping effect or an apical displacement cystocele. In its pure form, the displacement cystocele represents an eversion of the vagina with relatively well-preserved rugal folds. The loss of support of the lateral vaginal attachments can be unilateral or bilateral.
Preserved rugae with descent of the anterior vaginal wall suggests a defect of the connective tissue structures that support the vagina and urethra bilaterally at the lateral pelvic wall along the arcus tendineus fascia pelvis. This type of anterior segment defect is a displacement cystocele as is a cystocele from an upper vaginal vault prolapse. Descent of the upper vagina halfway between the ischial spines and hymen may easily be corrected by a surgical procedure directed only at the vaginal apex. Clinically, when the sulci disappear partially or completely, a paravaginal defect is coexistent. When one is evaluating the anterior segment, it is essential to determine whether the vaginal vault is partially or completely prolapsed, adding to the magnitude of a displacement cystocele. Additionally, with the vaginal vault replaced one must consider the extent of distention cystocele as well as the extent of “bridge” displacement cystocele by the vault prolapse. Any coexistent paravaginal defects are then and only then evaluated. To reposition the vaginal vault to the ischial spines with a ring forceps and label it a pure lateral displacement cystocele is an error in diagnosis. Yet, often our residents, referring colleagues, and patients call a posthysterectomy vault prolapse a “bridge” cystocele! Hence, the biggest imitator of a cystocele in a posthysterectomy patient is an apical vaginal segment prolapse.
It is essential that the surgical gynecologist contemplating surgical repair of anterior segment defects take into consideration that “bridge” forms of individual anterior segment defects rarely occur. As a general rule, anticipate a combination, that is, a “bridge” of the above types of defects.
Differential Diagnosis of Anterior Segment Defects
Examination of the patient with genital prolapse is facilitated by forceful Valsalva maneuver, especially in the standing position, in order to delineate defective vaginal sites that manifest themselves better under stress as well as the extent and magnitude of all defects. However, paravaginal defects are best evaluated in the lithotomy position initially at rest and then with forceful Valsalva maneuver to fully visualize descent of the anterior vaginal wall. Subsequent evaluation must analyze the site or sites of defects with repositioning of the sulci, as well as all other pelvic support defects if durable and long-term support of the anterior segment is to be obtained.
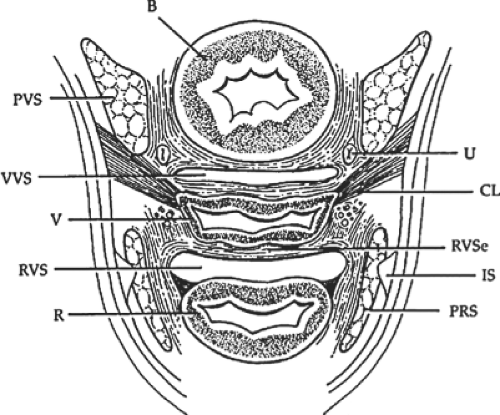
The importance of the damage caused by each component of the anterior vaginal segment may be noticeable at different levels within the anterior vaginal depth. Therefore, where are paravaginal defects along the vaginal depth? If we topographically divide the anterior vaginal segment into thirds, the paravaginal defects are in the middle third of the vagina. DeLancey examined vaginal support in cadavers that had previously undergone hysterectomy as well as cadavers with the uterus in place in order to study the relationship of vaginal support to uterine support, and vaginal support following hysterectomy. The structures that supported the vagina were divided into three levels corresponding to different areas of support: levels I, II, and III. Although these three levels of vaginal support are continuous, surgically we repair each of these three areas with different operations. We can almost select a surgical procedure and place it in one of these three levels.
One approach in evaluating patients with genital prolapse is for the initial assessment to be focused on the apical supports of the vagina. Descent of the vaginal apex or cervix suggests loss of connective tissue support between the vagina and the parametria, a level I defect. An undiagnosed partial vault prolapse may be spuriously treated surgically as a cystocele due to the telescoping effect of the anterior vaginal segment (from loss of apical support) in patients who have undergone hysterectomy. This apical defect is very frequently associated with a midline defect of the vaginal wall (distention cystocele).
The literature in reconstructive pelvic surgery has not clearly stated the incidence and coassociation of paravaginal defects in patients with primary and recurrent prolapse. Richardson reported that about 85% of the cystoceles with descent of the anterior vaginal segment in his patients and more than 95% of the cystourethroceles with stress incontinence were caused by a paravaginal defect. Therefore, 95% of his patients were treated with paravaginal defect repair.
Topographically dividing the anterior vagina into thirds permits evaluation of four distinct areas. This provides a useful paradigm that focuses on the defects and the surgical treatment that can be applied to their repair. As stated by DeLancey, these three levels of vaginal support are continuous with one another and therefore
interdependent. The midvagina, level II, relies on a well-supported upper vagina (level I) to prevent an apical displacement cystocele due to a telescoping effect of the anterior vaginal wall. As long as support of the upper vagina (level I) is intact, a “bridge” or anterior segment defect is a support defect at level II. Surgical correction of level I may be performed vaginally by unilateral or bilateral sacrospinous colpopexy—the Mayo-McCall culdoplasty—or an abdominal sacrocolpopexy. On the other hand, level III corresponds to the region of the vagina that extends 2 cm from the introitus to 3 cm above the hymenal ring and is fused with the surrounding structures. This is the area of the bladder neck—the urethrocele. In patients with total prolapse of the anterior vaginal wall and apex, urinary continence may be achieved by a “bridge” effect of the urethra. Reducing the cystocele and restoring the anatomic relationships of the anterior vaginal segment unmasks urinary incontinence.
interdependent. The midvagina, level II, relies on a well-supported upper vagina (level I) to prevent an apical displacement cystocele due to a telescoping effect of the anterior vaginal wall. As long as support of the upper vagina (level I) is intact, a “bridge” or anterior segment defect is a support defect at level II. Surgical correction of level I may be performed vaginally by unilateral or bilateral sacrospinous colpopexy—the Mayo-McCall culdoplasty—or an abdominal sacrocolpopexy. On the other hand, level III corresponds to the region of the vagina that extends 2 cm from the introitus to 3 cm above the hymenal ring and is fused with the surrounding structures. This is the area of the bladder neck—the urethrocele. In patients with total prolapse of the anterior vaginal wall and apex, urinary continence may be achieved by a “bridge” effect of the urethra. Reducing the cystocele and restoring the anatomic relationships of the anterior vaginal segment unmasks urinary incontinence.
Thus, the midportion of the anterior vaginal segment, level II, requires careful clinical evaluation because this is the site of both the midline distention cystocele and the lateral displacement cystocele (paravaginal defect). Therefore, a true cystocele develops because of midline damage to the anterior vaginal wall and/or to the lateral connective tissue supports of the bladder and arcus tendineus fascia pelvis. Attenuation and breaks in the connective tissue and/or the muscularis of the anterior vaginal wall within level II results in descent of the anterior vaginal wall into the vaginal canal as a space-occupying mass, that is, the cystocele. Since the distance from the left arcus tendineus fascia pelvis to the right arcus tendineus fascia pelvis is a fixed distance, any increase in the transverse anterior vaginal wall dimension along the length of level II will cause the anterior vaginal wall to bulge into the vaginal canal. Hence, an analogy can be drawn with respect to two fixed points such as clothesline poles and the clothesline between them.
Therefore, the diagnosis of a paravaginal defect requires the vaginal apex, level I, to be repositioned in its normal anatomic position or at the site where reconstructive surgery will place it; then and only then should level II be evaluated. Preoperatively repositioning the vaginal apex in patients with significant vault prolapse will avoid spuriously diagnosing an apical prolapse as a cystocele or as a paravaginal defect. Repositioning the vaginal apex is the initial step in correctly identifying the cause of level II defects and the complex nature of the pelvic floor defects. Huddleston evaluated patients with magnetic resonance imaging (MRI) preoperatively and then 4 to 30 days postoperatively after surgically correcting the paravaginal defects. This study very effectively identified the paravaginal defects preoperatively and the persistent uncorrected vault prolapse and level I defects postoperatively. The identification of the level I defects in the immediate postoperative MRI scans underscores the need to evaluate level II defects after level I has been repositioned, and if a level I defect exists it ought to be repaired.
Let us consider preoperative evaluation of a patient with an unreduced posthysterectomy vault prolapse to the level of the hymenal ring. The ring forceps may be utilized to reposition the sulci and elevate the vagina to the ischial spines. The vault prolapse and possible paravaginal defect is reduced and disappears as the most dependent portion of the vaginal apex is taken to the level of the ischial spines by the tip of the defect analyzer and as the sulci are elevated in the midvagina to the lateral pelvic sidewall, respectively. These findings may spuriously be diagnosed as a complete bilateral paravaginal defect. Alternatively, if the same patient was evaluated by initially reducing the apex of the prolapse with a disarticulated speculum, a sponge stick, or preferably a Scopette (rectal swab), this would immediately identify the vault prolapse as a level I defect. Then, with the vaginal apex repositioned, a lateral defect at level II may be evaluated by repositioning the sulci with the Baden vaginal defect analyzer or a curved ring forceps at rest and during Valsalva. When the vaginal apex is restored, the displacement cystocele from the apical prolapse is also noted, and any remaining cystocele is due to a midline defect. In clinical practice, the magnitude of pelvic floor genital prolapse will undoubtedly yield variations in the severity of anterior segment defects, that is, cystoceles. Multiple anterior segment site defects, “bridge” with midline distention, paravaginal defect, and apical descent components require meticulous preoperative evaluation.
Symptoms
The most common symptom of a displacement, distention cystocele, and paravaginal defect is an unwanted protruding bulge of anterior vaginal wall that is worse with the effects of gravity when the patient is standing or straining. There is a feeling of pelvic fullness and of “bridge.” Urinary incontinence may be a coexistent, troublesome symptom in patients with cystocele. Under normal conditions, the pelvic organs are constantly exposed to increasing intra-abdominal pressure and maintain their anatomic relationships. Increases in intra-abdominal pressure, as from coughing, sneezing, or changing position, and the intrapelvic relationship of the urethra to the posterior aspect of the pubic symphysis cause abnormal rotational descent of the bladder neck and flattening of the posterior urethrovesical angle if a relatively greater portion of the intra-abdominal pressure is transmitted primarily to the bladder, resulting in momentary but troublesome urinary incontinence. This is called urinary stress incontinence.
On the other hand, with large cystoceles a “bridge” may occur at the bladder neck, preventing urine loss with coughing and effective evacuation during micturition. When hypotonia of the bladder develops in such instances, normal detrusor tone is compromised, and excess urine from the incompletely emptied bladder may flow over into the urethra, a condition described as overflow incontinence. This may also leave a large volume of residual urine that may become stagnant and easily infected, giving rise to chronic cystitis.
In such cases, after colporrhaphy, the “bridge” effect is removed as the bladder base and vagina are repositioned. If there is insufficient urethral sphincter tone to maintain an intraurethral pressure greater than intravesical pressure under these circumstances, occult urinary incontinence may be unmasked, which has also been called iatrogenic urinary incontinence. For this reason, if one is performing an anterior colporrhaphy, preoperative testing with the cystocele reduced, provocative coughing with a full bladder, and preoperative urodynamic evaluation are essential, even in the absence of preliminary urinary stress incontinence symptoms.
The urethra penetrates the urogenital diaphragm, and condensations of the latter may be found alongside the urethra continuous with the urogenital diaphragm and extending anteriorly to the back of the pubis. These have various names, such as pubourethral ligament, puboprostatic ligaments of the female, and triangular ligament. However, they differ from the standard ligamentous anatomic descriptions in that they include elements of smooth and striated muscle and therefore are capable of expansion and contraction. This constitutes the upper suspensory system that helps to hold
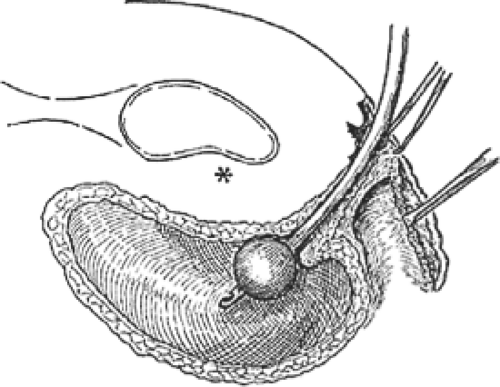
the urethra in place. The lateral sulcus of the vagina beneath the urethra and bladder is attached by a connective tissue bridge to the arcus tendineus of the pelvic diaphragm, which runs along the back of the pubis, takes origin from the medial surface of the obturator internus muscle, and is, in fact, the origin of the levator ani muscles. The attachments of the anterior vaginal wall thus support the vagina, urethra, and bladder, which the wall covers. It is clear that the urethra and vesicourethral junction are thus suspended from above by the urogenital diaphragm and supported from below by the anterior vaginal wall and lateral vaginal attachment, anatomic systems that are relatively distinct from one another (Fig. 3). It is possible for any of these systems to be damaged with resultant site-specific cystocele. The vesicovaginal space is a natural plane of cleavage between the wall of the vagina and the wall of the bladder and constitutes a potentially useful surgical space (Fig. 3). It is avascular in nature and extends from the vaginal vault to the vesicourethral junction and to the bladder pillars laterally. The vesicovaginal space permits independent function of the bladder and vagina. When this space has been lost as a consequence of laceration, fibrosis from infection, or the trauma of an operation, the ability for independent function of bladder and vagina is compromised. Subsequent bladder filling and distention as well as any bladder and vagina movement must thenceforth always occur simultaneously rather than independently. A similarly separate space between the vagina and rectum serving an analogous purpose is known as the rectovaginal space, whose dimensions and surgical usefulness as it pertains to posterior colporrhaphy are described later.
the urethra in place. The lateral sulcus of the vagina beneath the urethra and bladder is attached by a connective tissue bridge to the arcus tendineus of the pelvic diaphragm, which runs along the back of the pubis, takes origin from the medial surface of the obturator internus muscle, and is, in fact, the origin of the levator ani muscles. The attachments of the anterior vaginal wall thus support the vagina, urethra, and bladder, which the wall covers. It is clear that the urethra and vesicourethral junction are thus suspended from above by the urogenital diaphragm and supported from below by the anterior vaginal wall and lateral vaginal attachment, anatomic systems that are relatively distinct from one another (Fig. 3). It is possible for any of these systems to be damaged with resultant site-specific cystocele. The vesicovaginal space is a natural plane of cleavage between the wall of the vagina and the wall of the bladder and constitutes a potentially useful surgical space (Fig. 3). It is avascular in nature and extends from the vaginal vault to the vesicourethral junction and to the bladder pillars laterally. The vesicovaginal space permits independent function of the bladder and vagina. When this space has been lost as a consequence of laceration, fibrosis from infection, or the trauma of an operation, the ability for independent function of bladder and vagina is compromised. Subsequent bladder filling and distention as well as any bladder and vagina movement must thenceforth always occur simultaneously rather than independently. A similarly separate space between the vagina and rectum serving an analogous purpose is known as the rectovaginal space, whose dimensions and surgical usefulness as it pertains to posterior colporrhaphy are described later.
Findings on Physical Examination
The patient should be examined initially while in the lithotomy position, having been instructed not to empty the bladder before pelvic examination. The examination begins with the patient supine and at rest, then voluntarily squeezing the levator ani muscles to determine the extent to which this elevates the anterior sulcus and the bladder, and then voluntarily straining or bearing down. The examiner must evaluate all of the anterior vaginal wall segments for possible defects. While the patient is straining, careful note should be made of any coincident descent of the vaginal vault with or without the cervix, indicating the presence or absence of any displacement of the vagina and bladder, as described earlier. The rugae and character of the vaginal wall should be carefully noted as an estimate of its estrogen status. If the patient is postmenopausal and no contraindications exist, appropriate oral and vaginal estrogen replacement should be instituted. Any suburethral tenderness should be identified, and nodularity that might suggest a urethral diverticulum should be investigated. The patient should be asked to cough and any spontaneous leakage of urine noted with the structures in the presenting state, and then the patient should be repositioned in the anticipated postsurgical reconstructive position. If no leakage has occurred, the latter is repeated with the patient standing. Additional maneuvers consist of depressing the perineum, thereby stretching the levator ani, after which the patient is asked to cough again. Regardless of the event of urine loss, an estimate of urine volume at that examination time should be obtained by transurethral catheter or by ultrasound, and a concomitant urine specimen should be submitted for culture.
The patient should again be asked to stand, and the previously described examination repeated with specific emphasis on any eversion of the vaginal vault, enterocele, rectocele, and perineal defect.
Planning A Surgical Repair
Because it is impossible to make a patient feel better by operating on something that is asymptomatic to begin with, the goals of surgical therapy must be clear. These are (a) relief of symptoms, (b) restoration of structures to normal, and (c) restoration of function to normal. However, when a patient is to undergo a pelvic reconstructive operation for other indications, such as symptomatic prolapse of the uterus, a rectocele, or an enterocele, any cystocele, even one that is asymptomatic, should be repaired. Other than that, there is no indication for operating on an asymptomatic cystocele, except a demonstrated and sustained progression from year to year with additional areas of developing prolapse and symptoms. Conversely, when a patient demonstrates coincident weakness of the posterior vaginal wall or perineum, or both, a posterior colporrhaphy and perineorrhaphy as needed to help support the anterior vaginal wall and aid the long-term effectiveness of anterior colporrhaphy may be necessary. When there is an obvious coincident prolapse of the vaginal vault or uterus, or both, vaginal hysterectomy and vaginal vault fixation must be addressed to achieve the best long-term reconstructive result. The surgical reconstructive procedures should embrace techniques that are exacting and meet the specific needs of a particular patient. The hallmark of vaginal surgery is the development of vaginal wall flaps, using avascular planes between organs to reduce blood loss and minimize tissue trauma as well as to gain access to
potential pelvic spaces that provide exposure and facilitate the operative procedure. This surgical exposure permits an array of repairs of the underlying structures and organs as well as the vagina, including excision of damaged and overdistended vaginal wall, excision of enterocele, repair of cystocele and rectocele, repositioning of the vagina over the levator plate, and reconstructing the perineum and introitus. It is this exposure and flap generation that bridges plastic surgery with gynecologic reconstructive surgery.
potential pelvic spaces that provide exposure and facilitate the operative procedure. This surgical exposure permits an array of repairs of the underlying structures and organs as well as the vagina, including excision of damaged and overdistended vaginal wall, excision of enterocele, repair of cystocele and rectocele, repositioning of the vagina over the levator plate, and reconstructing the perineum and introitus. It is this exposure and flap generation that bridges plastic surgery with gynecologic reconstructive surgery.
When the vagina is used for surgical exposure during vaginal reconstructive surgery, the goal is to reconstruct a functional, pain-free, pliable, distensible, and well-supported structure. Vaginal reconstructive surgery requires the development of flaps, essentially full-thickness vaginal wall advancement flaps. The anterior and posterior vaginal walls are mobilized by careful dissection within the vesicovaginal and rectovaginal spaces, avoiding splitting the vaginal wall. The amount of full-thickness vaginal wall that is removed from the herniated damaged areas, the overall dimensions of the vaginal caliber and length, and the reconstruction of the perineum and introital aperture are often guided by the indications for surgery and the surgical judgment of the surgeon, which must also take into account repair of all defective vaginal sites. By far, the most frequent defect is that occurring in the midline structures (i.e., the urethra and anterior vaginal wall), so that most of the reconstructive surgical repair is concentrated in the midline. A systematic method for evaluating all the sites of damage is essential to surgical success. The goal of reconstructive surgery is to restore more normal anatomy, restore function, and relieve the symptoms. The massively everted vagina constitutes a completely decompensated vaginal organ with multiple defects in the midline at the vaginal apex/vault of the vagina as well as laterally. It is, in essence, a massive pelvic floor hernia composed of the bladder, small bowel, rectum, vagina, and, at times, the sigmoid colon.
When all of the areas of pelvic floor prolapse and anterior vaginal segment damage have been identified and evaluated, the surgeon must correlate the observations with the patient’s symptoms. Also, any other laboratory tests that may further explain any additional causes of urinary incontinence, such as detrusor dysfunction or other factors, must be figured into the surgical plan, and attempts must be made to correct these before a surgical repair of cystocele is undertaken.
The surgical technique of full-length anterior colporrhaphy described below presumes that the vaginal vault is located at or has been surgically replaced to a normal depth and that there is no coincident paravaginal defect. If any apical or lateral defects exist, performing only an anterior colporrhaphy will result in a foreshortened and narrow vaginal canal that will during coitus create a new symptom—dyspareunia.
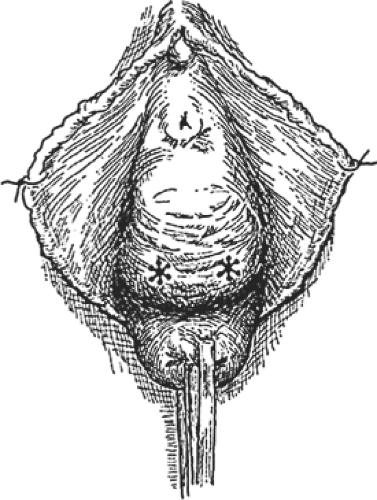
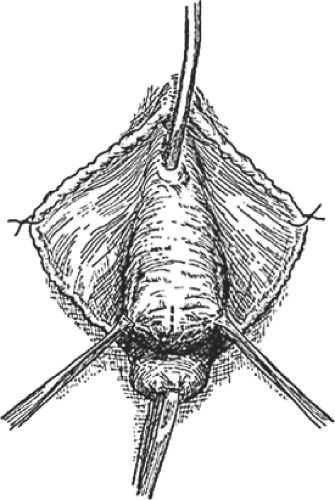
A distention cystocele as seen topographically is shown in Figure 4, and a sagittal section of a distention cystocele with rotational descent of the bladder neck is shown in Figure 5. Traction on the tenacula applied to the anterior and posterior aspects of the cervix brings the cystocele and cervix to the introitus and just outside the vagina. To avoid splitting the anterior vaginal wall and to facilitate dissection within the vesicovaginal space, Allis clamps are applied to the full thickness of the anterior vaginal wall overlying the cystocele, and the full thickness of the anterior vaginal wall is incised directly into the vesicovaginal space (Fig. 6). A depth of dissection away from the vesicovaginal space toward the vaginal epithelium splits the fibromuscular wall of the vagina. The dissection is performed with scissors, and the incision is carried to the anterior margin of the vesicovaginal space. The scissors dissection is continued to the distal urethral segment, creating a plane between the urethra and the vagina, which are normally fused one to another. This incision is continued to within 1 cm of the external urethral meatus (Fig. 7). The connective tissue capsule of the bladder is separated from the underside of the full thickness of the vagina with the scissors by sharp and blunt dissection (Fig. 8). This dissection mobilizes the bladder and urethra to the full lateral extent of the cystocele, at times as far as the medial margin of the pubis. The pubourethral ligamentous portion of the
urogenital diaphragm should be noted and their attachments preserved.
urogenital diaphragm should be noted and their attachments preserved.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree