Anterior and Low Anterior Resection of the Rectum
Robert D. Madoff
Genevieve B. Melton
Introduction
Anterior resection (AR) is most commonly performed for patients with high-to-mid level rectal tumors in the setting of rectal adenocarcinoma. AR can also be performed for benign conditions such as diverticular disease and other malignancies of the rectum. The technique discussed in this chapter is focused on achieving radical resection for rectal malignancies with proper total mesorectal excision (TME) and reconstruction. Both minimally invasive laparoscopic and open laparotomy techniques can be used for AR. Some surgeons also use a hybrid approach with laparoscopic abdominal exploration, splenic flexure, and left colon mobilization, followed by open proctectomy. A prospective trial is currently being conducted by the American College of Surgeons Oncology Group to determine the safety and efficacy of the laparoscopic approach for rectal cancer resection. This chapter does not include minimally invasive techniques with laparoscopy and robotic approaches for AR. Instead, we focus on AR performed using an open approach. The techniques described are also applicable with modifications to more extensive rectal cancer operations including vaginectomy or partial cystectomy but are beyond the scope of this chapter.
In the late 1970s, circular stapling devices were introduced, which made it possible for surgeons to safely perform anastomoses deep in the pelvis. In 1979, Heald reported that tumor deposits could sometimes be found in the mesorectum up to 5 cm distal to the primary rectal cancer tumor. During the same time period, Quirke and colleagues demonstrated the importance of obtaining negative circumferential resection margins in rectal cancer. Heald advocated and became the major proponent of TME, a technique of radical resection of the rectum with sharp dissection of the mesorectum (Fig. 1). Concomitant with this, trials using combined chemoradiotherapy were being conducted with the aim of decreasing local recurrence rates. Today, locally advanced rectal cancers (T3/T4 or N1/N2) are most commonly treated with neoadjuvant chemoradiation prior to radical resection.
Widespread use of TME has improved the oncologic outcomes after AR for rectal cancer. More recent studies addressing the issue of increased local recurrence rates following abdominoperineal resection (APR) compared to AR emphasize wide perineal dissection during APR to obtain a cylindrical specimen without the anatomic “waist” at the levator hiatus that is associated with an increased risk of a positive circumferential margin. For unclear reasons, APR with
end colostomy is still performed at high rates (30% to 60% of rectal cancer patients) in the United States.
end colostomy is still performed at high rates (30% to 60% of rectal cancer patients) in the United States.
A number of important factors must be considered in the decision between performing a restorative procedure with AR versus APR with permanent colostomy. This remains a complex assessment that must take into account oncologic and technical considerations, patient preferences, likely functional outcome, and surgeon experience. The level of the lesion and its relationship to the anal sphincters and pelvic floor (levators) is a primary consideration from a technical and oncologic standpoint. Additional oncologic factors include pretreatment staging, particularly imaging consistent with an advanced tumor or local invasion; relative response to neoadjuvant therapy; histologically aggressive tumors; and margin status. Patient factors, particularly a narrow pelvis and obesity, can add significantly to the technical difficulty of proctectomy. When performed for curative intent, both AR and APR involve TME to mobilize the rectum and mesorectum to achieve adequate margin clearance.
While some continue to advocate for a 2 cm distal mural clearance, recent reports demonstrate that smaller intramural margins may be adequate for low rectal cancers. This view has evolved from a historical understanding from cadavers advocating a 5-cm mural margin in colon cancer. Several retrospective studies in rectal cancer, however, demonstrated mural margins as small as 1 cm can be performed without increased rates of local recurrence. Although a 1-cm mural margin is adequate in most cases, a more generous margin should be considered for cancers with worse histological behavior, including signet cell, poorly differentiated, or mucinous histology. In addition to the mural margin, attention must be paid to the distal mesorectal margin. This is not an issue when dissection is carried to the level of the pelvic floor, as the entire mesorectal package is excised with rectal transection at this level. In contrast, because mesorectal tumor deposits can occur up to 5 cm distal to the caudal tumor margin, mesorectal transection for more proximal tumors should occur at least 5 cm distal to the lower tumor border, and the mesorectum must be divided perpendicular to the long axis of the rectum to avoid “coning in.”
Functional results for patients following AR vary significantly but tend to correlate with level of anastomosis. As a general principle, defecatory function becomes worse the more distal the anastomosis; similarly, anastomotic complication rates increase the more distal the anastomosis. Pelvic irradiation is also an important factor and tends to result in worse functional outcomes and more anastomotic complications. While most patients prefer to have a restorative procedure instead of a permanent end-colostomy, restorative procedures with sphincter preservation can be associated with poor function and quality of life. With a low colorectal anastomosis, the use of a colonic J-pouch should be considered for improving functional outcomes. Particularly for mid-to-low tumors or patients with poor body habitus, the decision to select AR versus APR will only be possible in the operating room when the rectum is completely mobilized.
Most AR resections are performed with a curative intent to achieve an R0 resection. AR will sometimes also be performed with curative intent in patients with isolated lung or liver metastases to obtain local tumor control and occasionally in cases of recurrent rectal cancer. In general, patients with a newly diagnosed rectal cancer require a full clinical assessment, including examination of the primary tumor, and thorough staging prior to any treatment. Important factors on history include pain with defecation or tenesmus, which may indicate anal sphincter involvement or a large tumor. It is also important to inquire about baseline bowel function, including bowel incontinence, as well as sexual and urinary function.
In cases where the tumor has not been biopsied, it is important to obtain histological confirmation of the presumed diagnosis with anoscopy (for distal tumors), rigid proctoscopy, or flexible sigmoidoscopy. A digital rectal examination is an invaluable tool for clinicians, particularly for mid or low tumors. Important features include tumor size, location from the anal verge and relative to the anorectal ring, orientation (i.e., anterior, posterior, left, or right), and mobility (i.e., assess as mobile, tethered, or fixed). For more proximal tumors, a flexible sigmoidoscopy or rigid proctoscopy should be performed for tumor assessment. Although there is no direct evidence, some surgeons prefer rigid proctoscopy as an assessment tool for determining distance and location of the tumor relative to the anorectal ring and anal verge. Particularly when a rectal cancer patient is referred from another clinician, it is imperative that a direct assessment of the tumor is performed by the surgeon to verify the lesion’s location, size, and consistency. This is helpful in verifying the correct preoperative work-up and treatment as well as gauging tumor response to neoadjuvant therapy. With use of neoadjuvant therapy, it can also be helpful to tattoo the tumor pretreatment as finding the tumor can sometimes be difficult in patients that have an excellent response to neoadjuvant therapy.
Patients also need a complete colonoscopy to rule out synchronous disease or other colonic abnormalities and a baseline carcinoembryonic antigen (CEA) level. A CT scan of the chest, abdomen, and pelvis to identify pulmonary and hepatic metastases should be performed. A chest CT is recommended because of the higher incidence of pulmonary metastases in rectal cancer patients compared to colon cancer patients.
Staging of the primary tumor is very important for rectal cancer, particularly for treatment assessment and to ascertain eligibility for combined modality chemoradiation. While CT scan can be helpful for assessing distant disease and gross pelvic abnormalities, it is inadequate for locoregional staging of the rectal tumor. Endorectal ultrasound (ERUS) and magnetic resonance imaging (MRI) are used most extensively for rectal tumor staging in the United States. Unfortunately, these tests are often not widely available and some centers lack adequate quality studies or sufficient experience to interpret these studies. In cases where adequate staging is not available, patients should be referred to experienced centers to obtain these studies.
ERUS is most helpful for staging early lesions for tumor wall depth (T1 or T2). ERUS is also moderately accurate as assessing lymph node status in the mesorectum (N-stage). Pelvic MRI with a rectal cancer-specific staging protocol has become increasing utilized for primary tumor staging. Most pelvic MRI studies are now done using high-resolution surface coils instead of an endorectal coil. Potential advantages of pelvic MRI are: (a) overall less operator dependent than ERUS, (b) better assessment of pelvic sidewall margin and other adjacent structures, (c) more anatomic information for operative planning, and (d) most likely better discernment of abnormal lymph nodes. MRI studies can help with identifying threatened circumferential resection margins, particularly tumor in the lateral pelvic sidewalls or obturator fossa. MRI can also help the surgeon visualize tumor location relative to the pelvic floor or to other pelvic structures.
While the use of neoadjuvant chemoradiotherapy for high rectal cancers is debatable, in most cases of advanced rectal adenocarcinoma in the mid or low rectum in the United States are treated with “long course” 6 to 8 weeks of chemoradiotherapy delivering 50.4 Gy along with 5-fluorouracil-based chemotherapy. After completion of this, surgical resection is performed 8 to 12 weeks later. This is in contrast to “short course” protocols administered primarily in Europe administering 25 Gy over 5 days followed by surgery 1 week later or protocols which forgo preoperative treatment in cases where TME alone appears adequate on imaging to clear the cancer. Neoadjuvant therapy has been demonstrated to decrease local recurrences but has not had a significant impact on overall survival in several large trials. Following surgery, adjuvant chemotherapy may be offered to address the risk of systemic recurrence in high-risk patients (node-positive on preoperative evaluation).
While the surgeon often plays a central role in the evaluation and treatment of rectal cancer patients, it is important to recognize the importance of interdisciplinary teams for optimizing treatment options and other decision making. This includes participation from medical and radiation oncologists for overseeing preoperative neoadjuvant treatment and experienced pathology and radiology staff dedicated to the interpretation of imaging studies such as pelvic MRI and gastrointestinal tissue specimens, respectively. Operative planning for distal or mid-level tumors should also include counseling and site marking for a potential stoma by an enterostomal therapist. In patients where it is unclear whether an APR or AR will be performed, both sides of the abdomen should be marked for either an end colostomy or a temporary diverting ileostomy, respectively.
Initial Setup
While recent studies have militated against the routine use of mechanical bowel preparation, we generally order a full mechanical bowel preparation prior to AR in order to avoid a colon full of stool distal to a diverting ileostomy. Epidurals are an excellent method for effective postoperative analgesia but may be contraindicated in some cases and require a devoted anesthesia staff for troubleshooting and follow-up. Both pharmacologic and mechanical thromboembolic prophylaxes are administered preoperative, as well as intravenous antibiotics within 30 minutes of the incision. A Foley catheter and an orogastric tube are placed after induction of anesthesia. Ureteral stents can also be useful, particularly in cases where the primary cancer appears to involve other organs, when the tumor is bulky, or in cases of recurrent rectal cancer.
Positioning should be performed carefully to minimize the potential for positioning injury. Buttocks should be brought to the edge of the table, and the legs placed into Yellow Fin® or Allen® stirrups in modified lithotomy position. Proper alignment includes the following elements: (a) hips slightly flexed and abducted, (b) feet flat within the stirrups, (c) creation of an imaginary line aligning the ankle, knee, and opposite shoulder, and (d) avoiding pressure along the lateral aspect of the leg.
Mobilization of Splenic Flexure
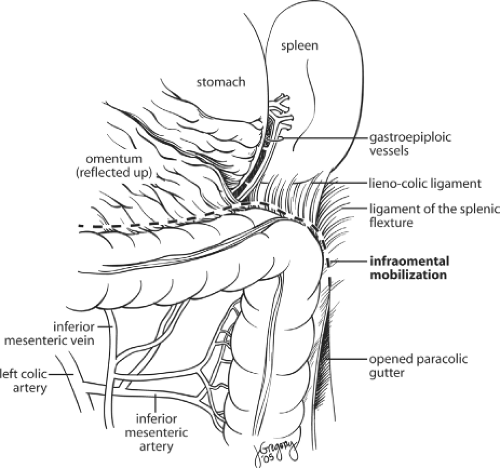
A midline incision is generally performed and the abdomen is explored to identify metastatic disease or other pathology. The intestines are packed into the upper abdomen. While some centers advocate the use of a “no-touch technique” with medial to lateral dissection with vascular division prior to lateral mobilization of the tumor, most surgeons perform lateral dissection first, by retracting the sigmoid colon medially and dividing the peritoneal attachments laterally along the white line of Toldt. The gonadal vessels and left ureter are identified and preserved in the process of separating the retroperitoneum from the left colon mesentery. This is an avascular retroperitoneal plane that should be extended superiorly and around the splenic flexure. The splenic flexure is then taken down fully. In cases where the flexure is high and not easily accessed, mobilization is facilitated by taking down the transverse colon attachments medially first and then continuing laterally to the splenic flexure. This can be accomplished by reflecting the omentum superiorly and accessing the lesser sac by dissecting superior and just adjacent to the colon wall (Fig. 2).
Division of Blood Supply
Once mobilization is complete, the inferior mesenteric artery (IMA) is exposed. The avascular space at the base of the mesentery near the IMA is identified, and the peritoneum on either side is incised. Ligation at the IMA at its origin is the preferred technique for some surgeons as this approach will result in increased lymph node harvest and possibly improved oncologic outcomes. In contrast, a lower ligation of the IMA distal to the left colic artery may prevent nerve injury which can occur at the IMA origin and can provide better collateral flow to the descending colon (Fig. 3). Evidence to date has not determined an optimal level of arterial ligation for rectal cancer, and both high and low ligations are considered acceptable oncologic options. After ligation of the arterial blood supply, the colon is divided at the descending-sigmoid colon junction with a linear cutting stapler along with division of the mesentery using clamps and ties. The descending colon should then be examined to ensure adequate mobilization to allow for a tension-free anastomosis. This will often require division of the inferior mesenteric vein as it descends within the colonic mesentery just below the pancreas, particularly for a low anastomosis. The descending colon is able to maintain its blood supply through the marginal artery and by collateral flow from the left branch of the middle colic artery and sometimes, when it is maintained, from the left colic artery.
Total Mesorectal Excision
TME aims to excise the rectum and surrounding mesorectum, which contains blood vessels and pararectal lymph nodes. Technically, this includes maintaining an intact “package” of visceral fascial envelope (fascia propria) around the rectum, along with the nodes located along the superior rectal and inferior mesenteric arteries. TME technically requires sharp dissection along the areolar plane located between the mesorectum at the visceral fascia (fascia propria) and the pelvic parietal (presacral) fascia (Fig. 4). The rectosigmoid is retracted anteriorly toward the pubis to expose the plane at the level of the pelvic inlet. At this point, the right ureter is identified and the peritoneal incision is extended to the pelvic brim and the plane is entered posteriorly in the retrorectal space.
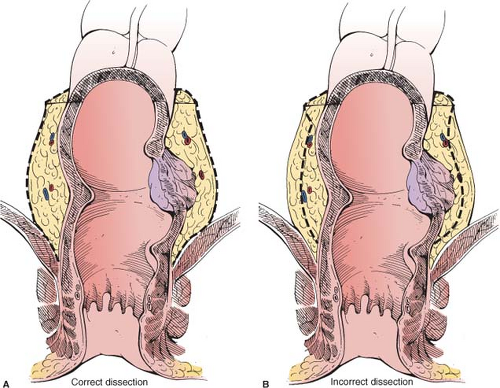
Dissection is facilitated with firm traction by the nonoperating hand and by the use of handheld retractors. Air enters the plane, which further facilitates dissection. In some cases, placing the patient in the Trendelenburg position can be helpful for exposure. The hypogastric nerves at the sacral promontory can be identified as they descend within the presacral space in a characteristic wishbone shape (Fig. 4). Preservation of these nerves is important for maintaining sexual and urinary function. Both ureters should also be identified and avoided. The dissection is continued posterior and lateral, and the endopelvic fascia envelope is maintained resulting in a bilobed mesorectum. As the dissection continues distally, Waldeyer’s fascia (rectosacral fascia) is divided at approximately the level of S3 (Fig. 5). If an AR is planned and appears technically feasible, the dissection is extended caudally to the levator hiatus. For cases known to require an APR, the dissection is stopped proximal to the levator muscles at the point that the mesorectum thins (approximately at S5) and the rectum changes course from posterior to anterior to avoid creating a “waist” in the specimen.
Anterior dissection can be performed after complete mobilization posterior and lateral or can be performed concurrently. It can be helpful to wait until most of the posterior dissection is complete and then to take the patient out of Trendelenburg or place him into reverse Trendelenburg to facilitate exposure. The peritoneum is incised at the rectouterine or rectovesicle pouch at the cul-de-sac starting anteriorly and then extending laterally to connect to the posterior-lateral
dissection (Fig. 6). When the tumor is anterior within the rectum, the dissection is carried anterior to Denonvillier’s fascia. If the tumor is posterior, the anterior dissection should be performed through Denonvillier’s fascia. Anterior dissection is facilitated by posterior retraction on the rectum and anterior retraction on the seminal vesicles or vagina. Special care should be taken to dissect close to the rectal wall anterolaterally at the level of the prostate to avoid injury to the parasympathetic nerve fibers required for erectile function. Although only encountered as a distinct vessel in a quarter of patients, the middle rectal artery runs along lateral dissection plane and occasionally will require formal ligation.
dissection (Fig. 6). When the tumor is anterior within the rectum, the dissection is carried anterior to Denonvillier’s fascia. If the tumor is posterior, the anterior dissection should be performed through Denonvillier’s fascia. Anterior dissection is facilitated by posterior retraction on the rectum and anterior retraction on the seminal vesicles or vagina. Special care should be taken to dissect close to the rectal wall anterolaterally at the level of the prostate to avoid injury to the parasympathetic nerve fibers required for erectile function. Although only encountered as a distinct vessel in a quarter of patients, the middle rectal artery runs along lateral dissection plane and occasionally will require formal ligation.
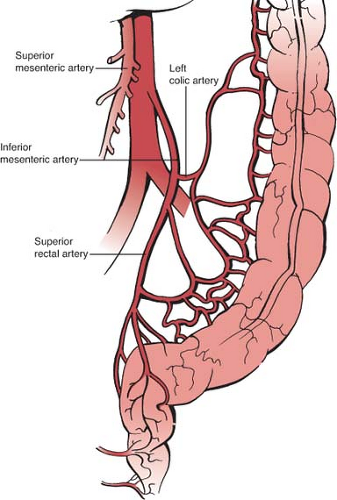 Fig. 3. Anatomic depiction of vascular ligation techniques. (A) “High ligation” refers to ligation of the inferior mesenteric artery (IMA) nears its origin. (B) “Low ligation” refers most commonly to ligation of the superior rectal artery.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|