Table 40-1. Vitamin-Dependent Metabolites in Patients with Vitamin B12 or Folate Deficiency
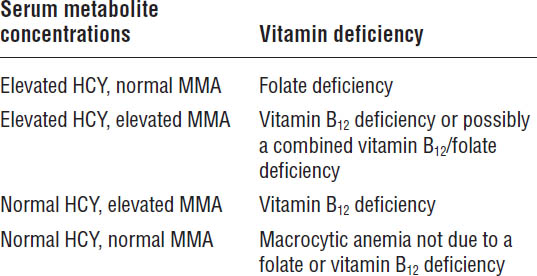
HCY, homocysteine; MMA, methylmalonic acid.
Additional signs and symptoms include the following:
■ Loss of vibratory sensation in lower extremities
■ Ataxia or vertigo
■ Glossitis
■ Muscle weakness
■ Neuropsychiatric abnormalities (e.g., irritability or emotional instability, dementia, psychosis)
Folic acid deficiency
Folic acid deficiency results in an increased serum homocysteine concentration with a normal serum methylmalonic acid concentration. Folate deficiency can occur sooner than vitamin B12 deficiency, with signs and symptoms of deficiency appearing within 6 months of folate depletion. Overall, signs and symptoms of folic acid deficiency anemia are very similar to vitamin B12 deficiency anemia, except that the neurological symptoms that may be present with vitamin B12 deficiency anemia are absent. Folic acid supplementation is given to pregnant women preemptively, because folate depletion can lead to neural tube birth defects (e.g., spina bifida) during fetal development.
Anemia of chronic kidney disease
As the name implies, this anemia occurs in patients with chronic kidney disease who require maintenance hemodialysis. The etiology for this type of anemia is multifactorial, including the effects of hemodialysis itself, which can lead to hemolysis, the removal of water-soluble vitamins (e.g., vitamin B12 and folate), and iron deficiency. Lack of EPO production also is a major factor. EPO is a hormone released from the kidney that stimulates the bone marrow to make new RBCs. Before diagnosis, other causes must be ruled out. A CBC usually will reveal a normochromic, normocytic anemia.
Bone marrow failure
In aplastic anemia, the bone marrow fails to produce multiple types of blood cells, resulting in anemia, neutropenia (decreased neutrophils), and thrombocytopenia (decreased platelets). About half of aplastic anemia cases are believed to be caused by drugs or chemicals. Drugs that cause aplastic anemia include chloramphenicol, felbamate, carbamazepine, and phenytoin.
Hemolysis
Hemolysis may be due to genetically inherited enzyme deficiencies. One common example is glucose-6-phosphate dehydrogenase (G6PD) deficiency. G6PD deficiency can lead to hemolysis, because RBCs deficient in G6PD are susceptible to hemolysis when exposed to certain oxidant drugs such as dapsone, sulfamethoxazole, and nitrofurantoin.
Treatment Principles and Goals
Iron deficiency anemia
In IDA treatment, the first goal is to normalize Hgb and Hct:
■ Hgb should increase 2 g/dL in 3 weeks.
■ Hct should increase 6% in 3 weeks.
■ Reticulocytosis usually will occur within 1 week.
If those indices do not improve within their respective time frames, the diagnosis should be reevaluated, and compliance with therapy should be confirmed. A second goal is to replenish iron stores. Although Hgb and Hct will return to normal within 1–2 months, iron therapy should be continued for 3–6 months after Hgb is normalized to replenish total body iron stores.
Megaloblastic anemias
Goals of vitamin B12 replacement
Hgb should increase within 1 week. If neurologic symptoms were present, they should improve within 24–72 hours. However, if vitamin B12 deficiency is longstanding, symptoms may not be relieved for several months. In some cases, some residual neurologic signs and symptoms may not resolve completely. Maintenance administration of vitamin B12 should continue for as long as nutritional intake, increased losses, or malabsorption is a problem.
Goals of folic acid replacement
RBC morphology will correct within a few days. Hgb will start to normalize within 10 days. Hct will return to normal levels within 2 months. Maintenance administration of folic acid should continue for as long as nutritional intake, increased losses, or malabsorption is a problem.
40-4. Drug Therapy
Iron Deficiency Anemia
Treatment consists of iron supplementation to achieve a total elemental iron dose of about 200 mg per day (Table 40-2). The total daily dose usually is divided into 2–3 dosing intervals throughout the day. The ferrous sulfate salt, which is 20% elemental iron, is the most commonly used and inexpensive salt form for iron therapy. Treatment of IDA with ferrous sulfate 325 mg three times daily for 3–6 months will be adequate for many patients.
Iron is best absorbed in the reduced (ferrous) form. Iron is maximally absorbed in the duodenum, primarily because of the acidic nature of the stomach. Vitamin C (ascorbic acid) may facilitate increased absorption of iron, although its clinical relevancy has been questioned. Additionally, orange juice may improve absorption. High-fiber foods, tea (rich in iron-binding tannins), coffee, and milk should be avoided during the administration of iron preparations. It is recommended that the iron product be taken on an empty stomach; however, most patients experience significant adverse GI effects necessitating that the drug be taken with either a snack or food. Some patients can tolerate only a product with a low elemental iron content (e.g., ferrous gluconate), a slow-release/sustained release preparation, or an enteric-coated iron product. However, the sustained release and enteric-coated products are less effective, because absorption is delayed because of slower dissolution, and iron is not available for absorption until the product has reached the jejunum or ileum, where iron absorption is decreased. If the patient is selected for one of these latter therapeutic options, then clinical recovery from the IDA would be expected to occur at a slower rate, and increased duration of therapy from 3–6 months to 6–12 months would be expected.
Table 40-2. Common Oral Iron Preparations Used To Treat IDA

Once the iron deficiency has been adequately treated, maintenance therapy may consist of a lower dose (e.g., ferrous sulfate 325 mg once daily or a multivitamin product that is enriched with iron) if the precipitating cause for the IDA is still evident (e.g., menses). Additionally, dietary intake of meat, fish, and poultry may be encouraged, because iron from vegetable, grain, and dairy sources is poorly absorbed.
Patient instructions (for oral supplementation)
■ Take iron supplementation 1–2 hours prior to a meal (on an empty stomach if tolerable).
■ If iron is intolerable on an empty stomach, take it with a small snack, but try to avoid dairy products or tea. Food can decrease the absorption of iron by 50%. Iron may be taken with an acidic fruit beverage such as orange juice to improve absorption.
■ Keep out of reach of children. Iron is a major cause of ingestion deaths in children.
■ Take iron 1 hour before or 3 hours after any antacids.
■ Some medications interact with iron. Please ask your health care provider or pharmacist before taking any new medications in combination with iron.
■ If constipation occurs, you may take over-the-counter docusate.
Adverse drug effects
The oral formulation primarily has GI effects:
■ Dark-colored stools
■ Constipation or diarrhea
■ Nausea or vomiting
Drug interactions
■ Antibiotics (tetracycline and quinolones): Iron binds to these antibiotics, preventing absorption.
■ Antacids: Iron needs an acidic environment for optimal absorption.
Intravenous (IV) iron preparations should be used only in the following cases:
■ Iron malabsorption with the inability to overcome the malabsorption with enteral iron supplementation
■ Oral noncompliance with severe anemia
■ Refusal of blood transfusion for severe anemia
■ Chronic kidney disease patients or cancer patients receiving human recombinant erythropoiesis stimulating agents
Patients with an active infection should not receive IV iron therapy. When iron is given intravenously, free iron concentration can easily exceed maximum transferrin saturation (unlike when given orally). The abundant free iron is then available to be used by various microorganisms for proliferation and may actually increase the virulence of the offending organisms.
Five IV iron products are available in the United States:
■ Iron dextran (INFeD and Dexferrum)
■ Ferric gluconate (Ferrlecit)
■ Iron sucrose (Venofer)
■ Ferumoxytol (Feraheme)
■ Ferric carboxymaltose (Injectafer)
Ferumoxytol was approved in 2009 for IDA in adult patients with chronic kidney disease. Ferric carboxymaltose was approved in 2013 for IDA of various etiologies in adult patients who cannot tolerate or who have not responded well to oral iron products.
Mechanism of action
Iron supplementation corrects the iron deficiency and enables Hgb to be synthesized at normal levels.
Published methods for calculating the parenteral dose or iron deficit differ. One method follows:
iron deficit(mg) = [0.3 × weight(lb)] × [(target Hgb – current Hgb)]/target Hgb * 100]
Iron dextran may be given intravenously in intermittent doses (e.g., 100 mg daily) or as a total dose infusion mixed in 250–1,000 mL of normal saline (0.9% NaCl) over 4–6 hours. Iron dextran formulations carry a black box warning about fatal anaphylactic reactions. A 25 mg test dose must be administered by slow IV push or intermittent infusion, and the patient must be observed for any adverse or allergic reactions for up to 1 hour after administration. This test dose is required before the patient can receive a larger therapeutic dose of iron dextran. The other IV iron products do not contain dextran and have a better safety profile; therefore, they do not require a test dose. Iron dextran is the only IV iron product approved for total dose infusion. Iron dextran also may be given intramuscularly (by Z-track injection to prevent potential skin staining) at doses up to 100 mg per injection. Iron dextran can be added to lipid-free parenteral nutrition solutions; however, it cannot be added to lipid-containing parenteral nutrition solutions, because iron is a trivalent cation and can disrupt the emulsification of lipids, resulting in oiling out or coalescence of lipid particles.
Ferumoxytol does not require dilution, and doses of 510 mg may be given as a rapid IV push at a rate of 1 mL/second (30 mg/second). It should not be given intramuscularly or subcutaneously. Ferumoxytol is approved to deliver 1,000 mg of IV iron in two doses. The second dose should be administered 5–8 days after the first dose. The IV iron formulations may have the following adverse effects:
■ Immediate reaction (within minutes of infusion)
• Malaise, urticaria, nausea, diaphoresis, headache
• Anaphylactic: Anaphylactoid reaction, hypotension, circulatory collapse. Premedication with antihistamines and corticosteroids may prevent anaphylaxis but is rarely done because of the low incidence of these reactions.
■ Delayed reaction (within 1–2 days after infusion)
• Myalgias, arthralgias, fever, flu-like symptoms
Monitoring parameters
■ Have reticulocytes, Hgb, and Hct increased?
■ Is the iron tolerable? (Tolerance will influence compliance.)
■ Is the patient improving symptomatically?
Kinetics
Bioavailability of oral iron preparations is increased in an acidic environment and is decreased by food.
Megaloblastic Anemias
Treatment of an existing vitamin B12 deficiency usually is done by the parenteral route; however, some clinicians have been successful with sublingual, intranasal, or high-dose oral therapy. Early and aggressive treatment is warranted, particularly if the patient has any neurologic signs and symptoms, because these adverse effects may not be completely reversible if the anemia has been allowed to persist for a substantial time.
A severe vitamin B12 deficiency (e.g., neurologic signs or symptoms) usually is corrected through intramuscular (IM) or subcutaneous vitamin B12 (cyanocobalamin) injection, although IV administration, if available, also is an option. Multiple dosing schemes are published in the literature. Some typical methods are as follows:
■ Initially 1,000 mcg IM, IV, or subcutaneous every day for 1 week, then 1,000 mcg IM, IV, or subcutaneous every week for 4–6 weeks
■ 1,000 mcg IM, IV, or subcutaneous every week for 4–6 weeks
■ 500 mcg intranasally or sublingually daily for 4–6 weeks (for less severe deficiency)
Once the deficiency has been corrected, various maintenance methods for prevention of recurrence exist depending on the etiology for the deficiency. If the etiology is low dietary intake such as with vegans (particularly ovo lacto-vegetarians), one simple maintenance solution is daily ingestion of a multivitamin that meets the Dietary Reference Intake for vitamin B12. If the etiology is malabsorption due to achlorhydria, pernicious anemia, chronic pancreatitis, drug-induced malabsorption, or gastric bypass therapy, either sublingual or intranasal administration (500 mcg weekly) is reasonable. Hot liquids or foods should be avoided for 1 hour before and after intranasal administration in an effort to avoid rhinorrhea and potentially reduce absorption. High oral doses of vitamin B12 may be used as maintenance therapy for some patients (e.g., 1,000–2,000 mcg daily is necessary to benefit from passive absorption). For patients with short bowel syndrome or severe malabsorption, the oral route should be avoided. The parenteral route also is an option (e.g., 1,000 mcg IM, IV, or subcutaneous every 1–3 months), although most patients would prefer sublingual or nasal administration.
Mechanism of action
Vitamin B12 supplementation allows for normal synthesis of the RNA involved in RBC synthesis.
Patient instructions
If injections are given at home, the patient or caregiver should be counseled on sterile injection techniques and proper needle disposal.
Adverse drug effects
Vitamin B12 supplementation can cause the following adverse effects:
■ Hyperuricemia or hypokalemia caused by increased synthesis of reticulocytes
■ Sodium retention
■ An expansion of the intravascular volume as a result of increased RBC synthesis, which can increase cardiac output and cause angina or dyspnea
■ Itching in 1–10% of patients
■ Diarrhea in 1–10% of patients
■ Anaphylaxis in < 1% of patients
Monitoring parameters
■ Monitor CBC. Is there an increase in Hgb? The hemoglobin should improve within 2 weeks. The anemia should correct within 1–2 months, although abnormalities in the blood smear may persist for several months.
■ Is the patient improving symptomatically (especially neurologic symptoms, if present)?
■ Methylmalonic acid concentrations should normalize within 1 month depending on the intensity of the repletion therapy.
Kinetics
Intrinsic factor is necessary for vitamin B12 absorption. It must be present for vitamin B12 to be transported optimally across the GI mucosa. Also, a small amount of passive diffusive absorption (about 1%) of vitamin B12 occurs, which is independent of intrinsic factor.
Vitamin B12 is bound in blood to transcobalamin II and converted in tissues to active coenzymes methylcobalamin and deoxyadenosylcobalamin.
Folic Acid Deficiency Anemia
Folic acid deficiency is corrected by administering 1 mg folic acid daily for 4 months. Five mg daily may be given if the patient has severe malabsorption. Once the underlying cause of the deficiency is corrected, folic acid supplementation may be discontinued. Long-term folate administration is necessary if the cause is not corrected, such as in hemodialysis, chronic drug therapy with sulfasalazine, or alcoholism.
Mechanism of action
Folic acid supplementation allows for normal RNA synthesis, which is involved in the synthesis of RBCs.
Patient instructions
■ Stress the importance of compliance with the regimen.
■ Women of childbearing age should be counseled to take a multivitamin containing folic acid, regardless of whether an anemia is present, to prevent neural tube birth defects.
Adverse drug effects
Fewer than 1% of patients have allergic reactions to folic acid.
Drug interactions
Folic acid may increase phenytoin metabolism.
Phenytoin, primidone, sulfasalazine, para-aminosalicylic acid, and oral contraceptives may decrease folic acid concentrations.
Chloramphenicol may blunt the response to folic acid.
Monitoring parameters
■ Is the RBC morphology normalizing?
■ Are the Hgb and Hct normalizing? An improvement in Hgb should occur within 2 weeks. The anemia should be corrected within 1–2 months.
■ Homocysteine concentrations should normalize within 1 month.
■ Is the patient complying?
Kinetics
Folic acid is a water-soluble B vitamin absorbed in the small intestine with peak concentrations occurring at 30 minutes to 1 hour.
Anemia of Chronic Kidney Disease
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


