Anemia, Bleeding, and Infection
LEARNING OBJECTIVES
• INTRODUCTION
The purpose of this chapter is to teach pharmacists how to evaluate patients for anemia, bacterial infection, bleeding, and adverse hematological drug effects. Management of anticoagulant therapy, including adjustment of doses, as well as medications that cause various hematological disorders, can be readily found in pharmacotherapy textbooks and other resources. Therefore, these topics are not covered in this chapter. Pharmacists in a variety of patient care practice environments are routinely required to evaluate patients’ complete blood counts to evaluate for potential adverse medication effects and response to treatment. Pharmacists working in anticoagulation clinics routinely monitor for evidence of bleeding due to overanticoagulation. Pharmacists also monitor responses of treatments for various anemias and infections. Finally, pharmacists are also asked to evaluate for the presence of drug-induced adverse effects to the hematologic system.
• INTERPRETING THE COMPLETE BLOOD COUNT
This review is not intended to be a comprehensive listing of all possible abnormalities, but focuses on the common hematological abnormalities that are typically seen by pharmacists in dealing with adult patients. The complete blood count (CBC) consists of three major components: red cells (RBC), white cells (WBC), and platelets. When evaluating the results of a CBC, consideration should be given to gender, ethnicity, and normal variation. Up to 5% of the population, without any disease, will routinely have values that are outside the “normal” values. In addition, different references cite small differences in normal values. Therefore, this review will use a common sense approach to CBC interpretation and easy to remember normal and abnormal values.
Red Cells
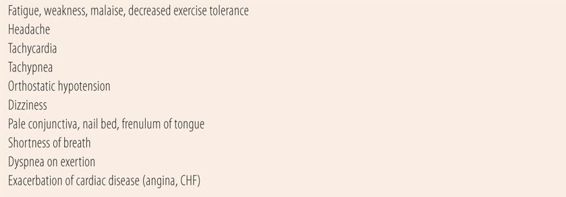
When looking at the red cell portion of the CBC, the first thing is to determine whether or not the patient is anemic. Anemia is defined as a decreased red cell mass, manifested as below normal levels of hematocrit (HCT) and red cell count (RBC). Hematocrit is the percentage of blood volume that comprises red blood cells. Values differ in adults by gender due to a combination of the erythropoietic effect of higher levels of testosterone in males, and regular blood loss due to menses in females. Normal values are 42% to 50% in males and 36% to 45% in females. Hemoglobin (HGB) is the oxygen carrying moiety within erythrocytes (RBC). Anemias are characterized by amounts of hemoglobin <13.5 g/dL in males or 12.0 g/dL in females. The red cell count (RBC) is done by instrument and normal values range from 4.5 to 5.9 × 106 in males and 4.1 to 5.1 × 106 in females. The normal values may be slightly higher for patients with chronic hypoxia such as patients with COPD, those living at high altitude or who are heavy smokers. Patients with anemia may have symptoms that are caused by physiological responses to poor oxygenation of the tissues, due to the lowered oxygen-carrying capacity of the blood (Table 25.1).
| TABLE 25.1 | Signs and Symptoms of Significant Anemia (Caused by Hypoxia or Hypovolemia) |
If the patient is anemic, the next step is to determine the type of anemia. There are three main types of anemia defined by the color and size of the red blood cell (Table 25.2). Cell size and color are determined by the red cell indices, which are mathematical manipulations of RBC, HCT, and hemoglobin (HGB) values. Mean corpuscular volume (MCV) is used to determine average red blood cell size. The MCV is calculated by dividing 10 times the HCT by the RBC count. Normal values are 80 to 100. Patients with a low HCT and a normal MCV are said to have a normocytic or normal red cell anemia. Patients with a low HCT and an MCV of less than 80 have a microcytic or small red cell anemia. Finally, patients with a low HCT and an MCV >100 have a macrocytic or large red cell anemia. The mean corpuscular hemoglobin (MCH) is the hemoglobin value × 10 divided by the RBC count. The mean corpuscular hemoglobin content (MCHC) is the hemoglobin × 100 divided by the HCT. Anemias with normal values for MCH or MCHC are termed normochromic anemias, because the amount of oxygenated hemoglobin that gives blood its red color is normal, while anemias with low MCH/MCHC levels are called hypochromic anemias due to lower levels of oxygenated hemoglobin, which results in less intense red color of blood.
| TABLE 25.2 | Classification of Anemias |
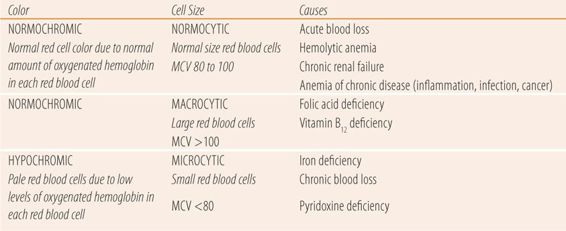
There are multiple causes of the three types of anemia. Normochromic, normocytic anemias have normal cell size and color and are caused by either destruction or sudden loss of red cells such as hemolytic anemia, acute blood loss, or by disorders of erythropoietin production/efficacy, usually classified as anemias of chronic disease. Causes of anemias of chronic diseases include chronic infections (HIV, hepatitis B/C, bacterial endocarditis, or osteomyelitis), autoimmune diseases (rheumatoid arthritis, Crohn disease, systemic lupus erythematosus), lymphomas, and chronic liver or renal disease. Hypochromic, microcytic anemias are primarily caused by iron deficiency due to poor nutrition or chronic blood loss. Finally, normochromic, macrocytic anemias are caused by vitamin B12 or folate deficiencies.
There are several important parameters that assist in the diagnosis or in monitoring the response to therapy. The first is the red blood cell distribution widths (RDW), which is a measurement of the variation in red blood cell size in the sample and is calculated by instrument. The normal value is 11% to 15%. It is high in anemias caused by iron, folate, and B12 deficiencies, and is primarily used in distinguishing iron deficiency anemias from other common causes of microcytosis such as thalassemia. Two terms are often associated with abnormal RDW. Anisocytosis means that on microscopic examination of blood there are a variety of red blood cell sizes on the peripheral smear (also measured by instrument) and is present in anemias due to iron, folate, and B12 deficiencies. Poikilocytosis means that there are a variety of shapes of red blood cells and is typically found in macrocytic anemias and in sickle cell anemia. The second important value is the reticulocyte count. Reticulocytes are immature red blood cells and the normal value is 0.5% to 2.5%. In patients with anemias due to iron, folate, and B12 deficiencies, using very small amounts of the deficient vitamin or mineral for 5 to 7 days will cause the reticulocyte percentage to go to double digits. Before the days of accurate serum levels of folate, B12, and iron, giving very small amounts and getting a marked response was used diagnostically (Castle test to distinguish folate from B12 deficiency). Failure to respond with double digit reticulocytosis indicates that there is some other cause for the anemia than the vitamin or mineral deficiency. Today, the reticulocyte count is used primarily to monitor the efficacy of replacement therapy. The reticulocyte count can also be reported as the reticulocyte index (RI) or corrected reticulocyte count, which is more accurate, since it adjusts the reticulocyte percentage by multiplying it by the actual HCT divided by 45.
Other Helpful Tests to Evaluate Anemias
Iron deficiency anemia is the most common form of anemia. Among nutritional causes, poor dietary intake and pregnancy (which increases demand for iron) are common causes. Chronic blood loss is also a common cause. Above average, monthly menstrual blood loss and upper gastrointestinal bleeding are the most common etiologies. All red blood cell parameters are below normal except for RDW, which many times is elevated. In addition, a serum ferritin level of less than 10 μg/mL, is the most accurate indication of iron deficiency, reflecting severely depleted iron stores. While not routinely used in the diagnosis, serum transferrin levels (<30%) and serum iron levels (<50 μg/mL) will be decreased. Total iron-binding capacity may be increased (>250 μg/mL) in an attempt to capture more iron as it is absorbed into the blood stream from the gastrointestinal tract.
Macrocytic anemias due to vitamin B12 or folic acid deficiencies, may have reduced or normal hemoglobin levels even though their MCV is >100. Generally, low B12 and folate blood levels are found depending on the specific deficiency. However, serum folate levels vary based on folate intake; therefore, RBC folate levels are a more accurate indication of folate status. Serum folate levels <5 μg/L and RBC folate levels <166 μg/L are diagnostic of folate deficiency. Similarly, about 5% of patients with B12 deficiency will have a normal or near normal serum B12 level due to the presence of other B12-like substances. Therefore, in most patients with B12 levels in the low normal range, serum methylmalonic acid (MMA) and homocysteine levels will be drawn. In B12 deficiency, both will be elevated. They can also be used to confirm folate deficiency since the MMA level will be normal with an elevated homocysteine level. Both macrocytic anemias will have megaloblasts (large immature red cells). Because of that finding, B12 and folate deficiency anemias are sometimes referred to as megaloblastic anemias. In both deficiencies, hypersegmented neutrophils (>6 nucleated segments) are often present. In addition to an increased RDW and anisocytosis, macrocytic anemias may also present with poikilocytosis.
Most macrocytic anemias are caused either by low intake of folate or B12, or by diseases that cause malabsorption of nutrients (including B12 and folic acid) from the intestine, e.g., tropical sprue, pernicious anemia. Pernicious anemia is a classical malabsorption syndrome that causes B12 deficiency. The absorption of B12 requires an active process that involves both gastric acid and a protein called intrinsic factor, which are both produced by the parietal cells of the gastric lining. An autoimmune process attacks and destroys the parietal cells, leading to low B12 levels along with achlorhydria and a lack of intrinsic factor that leads to a macrocytic anemia. In addition, B12 deficiencies, like pernicious anemia, have other nonhematological complications including irreversible peripheral neuropathy, cognitive decline, and cardiac muscle damage, leading to congestive heart failure. Therefore, it is critical to accurately obtain a specific cause for a macrocytic anemia, since giving folic acid supplements to a patient with a B12 deficiency may partially correct or “mask” the anemia, but allow the neurological and cardiovascular damage to progress. That is why the amount of folate in over-the-counter vitamins is limited to 400 μg, to prevent the masking of pernicious anemia or B12 deficiency. Another cause of macrocytic anemia of interest to pharmacists is prescription medications. Traditional anticonvulsants such as phenytoin and carbamazepine, through induction of CYP450 enzymes, and methotrexate by direct antagonism can cause folate deficiencies. Chronic use of proton pump inhibitors, such as omeprazole and the diabetic medication metformin, have been reported to cause B12 deficiencies.
White Cells
There are five major types of white blood cells. Neutrophils, also known as segmented neutrophils (segs) or polymorphonuclear cells (PMNs or polys), are the primary defense against bacterial infections since they ingest and digest foreign proteins. Immature neutrophils are called bands or stabs and are normally not in circulation. Lymphocytes are the primary defense against viruses, fungi, mycobacterium, and malignant neoplasms. Lymphocytes are also involved in producing antibodies to a variety of viruses and bacteria, which subsequently provide immunity against those organisms. Monocytes are in circulation only 16 to 36 hours before they transform into tissue macrophages, which are active for months to years. They are the garbage collectors, ingesting foreign proteins, old red cells, plasma lipids, and plasma proteins. Macrophages are also an important component of both cell-mediated and antibody-mediated immune processes. Eosinophils are associated with IgE-mediated immune processes such as asthma, allergic rhinitis, atopic dermatitis, and urticaria, as well as with parasitic infections. The function of basophils is not clearly known, but they are thought to participate in both immediate and delayed hypersensitivity reactions. All precursors of white cells are produced in the bone marrow. PMNs continue to differentiate in the bone marrow, while lymphocytes differentiate in lymphatic tissue. Granulocytes (neutrophils, basophils, and eosinophils) have a short duration of existence in serum, ranging from 1 to 5 days. On the other hand, lymphocytes persist in serum for almost 90 days.
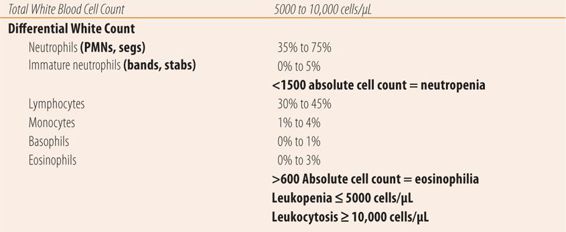
The white blood count consists of a total white blood cell count (WBC) and when the total white count is outside of normal the laboratory will automatically do a differential white count, which consists of measuring the percentages of each of the five types plus bands or stabs. Some facilities require a specific request for a differential count if the total white count is normal. However, most current automated instruments will automatically do a differential white blood cell count. Unfortunately, each process has its own normal range, which leads to a variety of normal values. For example, references for the lower limit of normal for the total white cell count can range from 4000 to 5000 cells/microliter (μL), and the upper normal levels range from 10,000 to 11,500 cells/μL. For the examples and exercises, 5000 to 10,000 cells/μL will be considered normal. Some conditions require an absolute cell count of each type for diagnostic purposes. To calculate the absolute count of a particular cell type multiply the percentage (as a decimal value) by the total white blood cell count. For a total white cell count of 8500 cells/μL and 4% eosinophils (0.04), the absolute count of eosinophils is 340. Unfortunately, reference values may also vary for several absolute counts. For example, the definition of eosinophilia (above normal numbers of eosinophils) ranges from 350 to 600 cells/μL. For the examples and exercises in this chapter, >600 cells/μL will be used to determine the presence of eosinophilia. In practice, most results from commercial laboratories list normal values for their procedures or tests. Abnormal values are indicated by an additional symbol, an H for higher than normal values, and L for lower than normal levels. See Table 25.3 for normal values and definitions. Also somewhat confusing is the relationship between the percentages of each cell type in the differential count and the absolute cell count. For example, during bacterial infections, the absolute neutrophil count substantially increases, while other cell type counts remain constant. In the differential count, the percent of neutrophils will rise dramatically, along with the absolute neutrophil count, while the percent of the other cell types, particularly the lymphocytes, will fall to below normal levels even though the absolute cell count is unchanged.
| TABLE 25.3 | Normal White Blood Cell Counts |
Using the WBC Count to Confirm the Diagnosis of Bacterial Infections and Monitor Treatment Efficacy
Bacterial infections induce an elevated total white count (leukocytosis) with a predominance of neutrophils. When neutrophils are released from the bone marrow, most end up adhering to the endothelial walls of the blood vessels, and normally the CBC measures only those neutrophils not adhering to the endothelium. When a bacterial infection occurs, the original responding neutrophils attack the bacteria, causing a release of chemotactic factors that cause the neutrophils adhering to the vascular endothelium to be liberated into the blood and travel to the site of infection. Those same chemotactic factors stimulate the bone marrow to release stored mature neutrophils. In a bacterial infection, these two factors cause the total white blood cell count to rise above 10,000 cells/μL and the combined percentage of mature and immature neutrophils to exceed 80% of the total white blood cell count. If a bacterial infection is severe or long standing, the marrow releases immature neutrophils into the blood stream to help battle the infection. Historically, this is called a left shift. A left shift is present when the total WBC count indicates bacterial infection (>10,000 WBC + >80% of WBC are PMNs, stabs, or bands) plus the percentage of immature neutrophils (bands/stabs) rise to double digits (≥10%). The total WBC count and differential can also be used to monitor the efficacy of antibiotic therapy and the resolution of the bacterial infection. As the neutrophils, with the aid of an antibiotic, successfully fight the bacterial infection, the total WBC count, absolute neutrophil count, and percentage of neutrophils will return toward normal, along with the percentages of the other cell types in the differential white blood cell count.
Other Causes of Leukocytosis
When the total WBC count rises above 12,000 cells/μL, and there is no evidence of bacterial infection, consideration must be given to the possibility of leukemias and lymphomas. The specific diagnosis requires a peripheral blood smear examined under the microscope and several other specific tests that are beyond the scope of this textbook.
Drug Induced Decreases in Red and White Blood Cells
Many medications have adverse effects on the bone marrow. Obviously, most cytotoxic cancer chemotherapy, which interferes with all rapidly growing cells, also negatively impacts the production of white blood cells in the bone marrow. Other medications have the same impact, but through different mechanisms ranging from pharmacological side effects to hypersensitivity or idiosyncratic reactions. Pharmacists need to monitor patients on medications with potential bone marrow adverse effects to prevent infectious complications. Granulocytes (neutrophils, basophils, and eosinophils) are the most susceptible to the toxic drug effects due to their short half-lives in the blood compared to lymphocytes and macrophages, which have much longer half-lives in tissue and blood. Eventually, they too will be impacted by continuous exposure to a bone marrow toxin. Since neutrophils are so important to defend against bacterial infections, we focus on their absolute counts. Neutropenia is defined as an absolute count of less than 1500 cells/μL. Once the levels drop below 500 cells/μL, normal bacterial flora in the oral cavity, gastrointestinal tract, and other parts of the body can cause serious infections. Granulocytopenia is a below normal number of neutrophils, basophils, and eosinophils. Agranulocytosis indicates granulocytopenia, plus the lack of production of granulocytes in the marrow and requires a bone marrow biopsy for diagnosis. Agranulocytosis carries a much more ominous prognosis than granulocytopenia, so care must be exerted not to use the terms interchangeably. Similarly, pancytopenia indicates below normal numbers of red blood cells, white blood cells, and platelets in the blood, whereas aplastic anemia refers to a pancytopenia, plus the lack of bone marrow production of all three formed elements in the blood and requires a bone marrow biopsy for diagnosis. With medications that cause decreases in the number of granulocytes, the absolute counts of the granulocytes will fall, along with their percentages of the total white cell count. The absolute counts of lymphocyte and monocytes, because of their longer half-life in serum, will remain unchanged, but the percentages will markedly increase due to the decrease in the absolute number of granulocytes.
Platelet Count
The final formed element of blood made by the bone marrow, platelets, is important to the blood clotting process, especially in arteries. In atherosclerotic cardiovascular disease (strokes and myocardial infarctions), platelet aggregation starts the infarction process. We use drugs like aspirin that interfere with platelet aggregation to prevent or reduce the risk of those events. These platelet inhibitors can also cause abnormal bleeding. While normal values can vary by lab, 150,000 to 400,000/μL is a representative normal range. Values below normal (thrombocytopenia) increase the risk of bleeding and values above normal (thrombocytosis) tend to increase the risk of clotting. In addition to the numbers of platelets, there are also tests of platelets’ ability to aggregate. Most tests are primarily used for research purposes. While the mechanism of action of aspirin is known, there are no practical methods for measuring its effect on platelet aggregation. However, for antiplatelet medications that primarily impact P2Y12, there are now reliable “point-of-service” tests that show promise in helping gauge effectiveness and adjusting doses of clopidogrel, prasugrel, and ticagrelor.
• MONITORING ANTICOAGULATED PATIENTS FOR BLEEDING
Anticoagulants are used to prevent pulmonary embolisms, myocardial infarctions, strokes, deep vein thrombosis, and venous thromboembolism. While monitoring patients on anticoagulants, the pharmacist checks for signs and symptoms of blood clots (stroke, pulmonary embolism) to evaluate the efficacy of the anticoagulant. In addition, some agents have specific tests and procedures that help pharmacists measure optimal anticoagulation and adjust doses. Finally, anticoagulants can also cause bleeding if given in higher doses or to patients with enhanced sensitivity to their effects. Therefore, it is important that pharmacists understand how to evaluate patients for bleeding, the major adverse effect of these medications.
The easiest way to conduct an interview for potential symptoms of bleeding is a modified review of systems (ROS) process (Table 25.4). If lab values indicate overanticoagulation, then a complete ROS should be done. If anticoagulation parameters are normal, then an individualized, but briefer version based on previous patient experiences and education is indicated. During the first few follow-up visits after initiation of anticoagulation therapy, a complete ROS should be done since it also serves as an educational process to teach patients what to look for as symptoms of bleeding complications. What the pharmacist focuses on are changes or new symptoms that might indicate potential bleeding complications.
| TABLE 25.4 | Evaluating Hemorrhagic Complications of Anticoagulants |

Head and Neck
One of the most common signs of bleeding is bleeding of the gums during and after brushing the teeth. Also bleeding from the nose can also be an indication of excessive anticoagulation. Visual changes and decreased visual acuity may indicate a hyphema (bleeding into the anterior chamber of the eye). Subconjunctival hemorrhages can also occur more readily in anticoagulated patients. Both of these are usually associated with trauma to the surrounding area or violent movement of the head, e.g., during sneezing. New onset headaches can indicate a hemorrhagic cerebrovascular accident, an intracranial or subdural bleed, or hypoxia in a severely anemic patient and warrant a neurological examination. Finally, check for paleness in the conjunctiva, frenulum of the tongue, and lips as a sign of significant anemia.
Skin
Another common site for detecting bleeding due to excessive anticoagulation is the skin. Excessive bleeding from cuts, e.g., shaving in males, may indicate overanticoagulation. Similarly, new onset of easy bruisability (bruising due to nominal trauma) or large deep bruises from minimal trauma may be an indication of overanticoagulation. Finally, bleeding just under the skin may warrant further investigation. Many times, it may be hard to distinguish the cause of subepidermal bleeding, especially in elderly patients, in whom it can occur naturally because of increased fragility of small arteries and veins due to aging. Similar effects can be caused by newly added medications, such as angiotensin-converting-enzyme inhibitors or nonsteroidal anti-inflammatory medications, which have mild anticoagulant effects of their own. Like the conjunctiva and tongue, the nail beds can be checked for loss of color, which represents significant anemia.
Gastrointestinal Tract
In patients with a history of peptic ulcer disease or on medications that may be ulcerogenic (corticosteroids, NSAIDs), checking for signs and symptoms of bleeding from the gastrointestinal (GI) tract is important. Symptoms such as heartburn and very dark stool may be an indication of upper GI tract bleeding. Large quantities of upper GI bleeding may cause melena (black tarry stools) or hematemesis in the form of bright red blood or coffee-ground vomitus. Pharmacists should be vigilant, especially in older patients, for a retroperitoneal bleed, which may start out as fullness or bloating, and eventually lead to more severe abdominal discomfort and pain. Finally, hematochezia or rectal bleeding of bright red blood can arise from the sigmoid colon or rectum or anal fissures, in patient with hemorrhoids, can be due to excessive anticoagulation.
Genitourinary (GU) Tract
Hematuria can be a sign of bleeding due to excessive anticoagulation in both genders. Microscopic amounts of hematuria cannot be detected by noting any change in color of the urine. However, small amounts of bleeding may present as pink, red, or red-brown stains on underwear or toilet paper. Gross hematuria may change the urine to colors ranging from pink to reddish brown depending on the amount of blood and the concentration of bilirubin by-products (urobilin) that cause urine to be yellow. In menstruating females, increased volumes and/or duration of menses can be an indicator of excess anticoagulation.
Miscellaneous
Trauma to joints, in addition to visible hematomas (bruises), may also cause hemarthrosis (bleeding into the joint) in anticoagulated patients. Knees and elbows are the most commonly affected. Pain, swelling, warmth, stiffness, and/or reduced range of motion are signs of bleeding into the joint space. In patients with COPD, hemoptysis can occur.
• KEY REFERENCES
1. Tefferi A, Hanson CA, Inwards DJ. How to interpret and pursue an abnormal complete blood count in adults. Mayo Clin Proc. 2005;80:923-936.
2. Kaferle K, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician. 2009;79:203-208.
3. Bryan LJ, Zakai NA. Why is my patient anemic? Hematol Oncol Clin North Am. 2012;26:205-230.
4. Pang WW, Schrier SL. Anemia in the elderly. Curr Opin Hematol. 2012;19:133-140.
5. Cerny J, Rosmarin AG. Why does my patient have leukocytosis? Hematol Oncol Clin North Am. 2012;26:303-319.
6. Reagan JL, Castillo JJ. Why is my patient neutropenic? Hematol Oncol Clin North Am. 2012;26:253-266.
7. Bhatt V, Saleem A. Review: drug-induced neutropenia—pathophysiology, clinical features and management. Ann Clin Lab Sci. 2004;34:132-137.
8. Carey PJ. Drug-induced myelosupression, diagnosis and management. Drug Saf. 2003;26:691-706.
9. Wong EY, Rose MG. Why does my patient have thrombocytopenia? Hematol Oncol Clin North Am. 2012;26:231-252.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



