Chapter 21 Phenols and phenolic glycosides
Phenols probably constitute the largest group of plant secondary metabolites. Widespread in Nature, and to be found in most classes of natural compounds having aromatic moieties, they range from simple structures with one aromatic ring to highly complex polymeric substances such as tannins and lignins. Phenols are important constituents of some medicinal plants and in the food industry they are utilized as colouring agents, flavourings, aromatizers and antioxidants. This chapter mainly deals with those phenolic classes of pharmaceutical interest, namely: (1) simple phenolic compounds, (2) tannins, (3) coumarins and their glycosides, (4) anthraquinones and their glycosides, (5) naphthoquinones, (6) flavone and related flavonoid glycosides, (7) anthocyanidins and anthocyanins, (8) lignans and lignin. The biosynthetic origin of some of these compounds involving the shikimic acid pathway is shown in Fig. 21.2. Phenols may also have aromatic rings derived by acetate condensation (Fig. 18.9.).
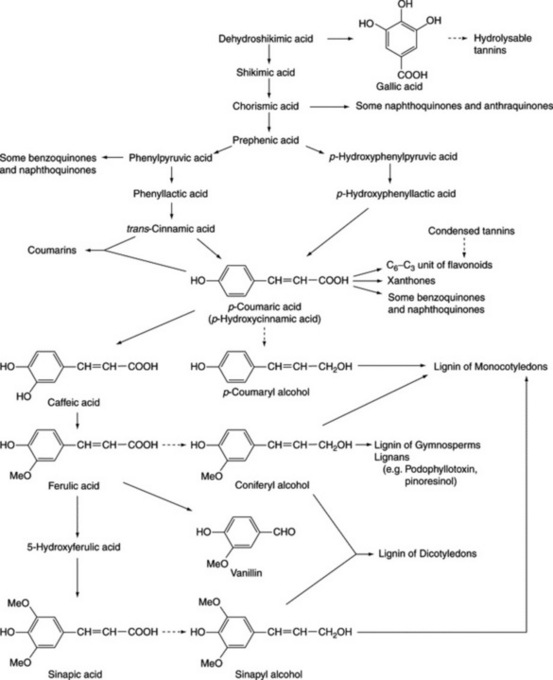
Fig. 21.2 Phenolic compounds originating from shikimic acid (see Fig. 18.8 for details of shikimic acid pathway).
SIMPLE PHENOLIC COMPOUNDS
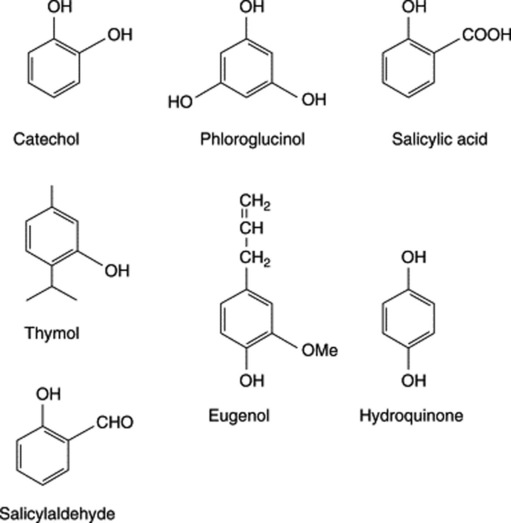
The phenolic compounds in this group often also possess alcoholic, aldehydic and carboxylic acid groups; they include eugenol (a phenolic phenylpropane), vanillin (a phenolic aldehyde) and various phenolic acids, such as salicylic, ferulic and caffeic acids. Glycoside formation is common, and the widely distributed glycoside coniferin and other derivatives of phenolic cinnamic alcohols are precursors of lignin. Some of the best-known simple phenolic glycosides are listed in Table 21.1.
Table 21.1 Examples of phenolic glycosides.
| Name | Examples of sources | Products of hydrolysis |
|---|---|---|
| Salicin | Salix and Populus spp. Viburnum prunifolium | Salicyl alcohol, glucose |
| Populin (benzoyl-salicin) | Populus tremula | Salicyl alcohol, benzoic acid, glucose |
| Arbutin | Ericaceae and Rosaceae | Hydroquinone, glucose |
| Phloridzin | Rosaceae, including spp. of Malus | Phloretin, glucose |
| Trilobatin | Malus, Spiraea | Phloretin, glucose |
| Coniferin | Coniferae | Coniferyl alcohol, glucose |
| Gaultherin | Gaultheria, Betula and Monotropa | Methyl salicylate, primeverose |
| Syringin | Particularly in Oleaceae | Methoxyconiferyl alcohol, glucose |
| Glucovanillin | Vanilla spp. and some Gramineae | Vanillin, glucose |
| Gein | Geum spp. | Eugenol, vicianose (glucose + arabinose) |
| Glucogallin | Rheum spp. | Gallic acid, glucose |
| Hamamelitannin | Hamamelis virginiana | Gallic acid (2 mols), hamamelose |
MEADOWSWEET
Meadowsweet BP/EP, Filipendula BHP 1983 consists of the dried flowering tops of Filipendula ulmaria (L.) Maxim. [Spirea ulmaria L.], family Rosaceae.
Among the complex mixture of structures in the powder the following can be noted: leaves and sepals having lower epidermis with slightly sinuous anticlinal walls, anomocytic stomata and cluster crystals of calcium oxalate up to 40 μm diameter in the mesophyll; papilose epidermis of petals; pollen grains with three pores and a smooth to slightly pitted exine; numerous trichomes, occasionally glandular with a one- to three-celled stalk and multicellular head with brown contents but principally clothing trichomes of various size, often twisted together; vascular tissue of the stem and veins.
Constituents
The BP/EP requires a minimum concentration of 0.1% for the steam volatile fraction of Meadowsweet; the flowers have recorded higher values. The major component of the oil (up to ca 70%) is salicylaldehyde (Fig. 21.1) together with methyl salicylate, benzaldehyde, benzyl alcohol, and smaller amounts of other components such as vanillin. In 1839, Löwingand and Weidmann, working on meadowsweet, were the first to report salicylic acid as a natural product. Other constituents of the drug are the phenolic glycosides gaultherin (Table 21.1) and spiraein (salicyl alcohol + primerose), various flavonoids, e.g. hyperoside (Fig. 21.18), tannins and mucilage.
WILLOW BARK
Willow bark is a source of salicin (Table 21.1), a phenolic glycoside now seldom used but generally regarded as the natural forerunner of aspirin. The composition of the glycoside mixture is variable in the bark depending on species, age of bark and time of collection. The latter is usually made in spring when the bark is easily removed from the branches. Other phenolic glycosides are salicortin (an ester of salicin), acetylated salicin (fragilin) and salicortin. Salicin is easy to prepare (see 15th edition of this book) and is a suitable compound with which to introduce students to this class of glycoside.
Flavonoids of the bark (to over 4%) include the 5- and 7-glucosides of naringenin, isoquercitrin and chalcone (see Fig. 21.18). Tannins are of the condensed types (q.v.).
HOPS
Hops are the dried strobiles of Humulus lupulus L. (Cannabinaceae). Only the pistillate plants are cultivated, large quantities being produced in England (particularly Kent), Germany, Belgium, France, Russia and California. The strobiles are collected, dried in kilns and pressed into bales known as ‘pockets’. They are sometimes exposed to the fumes of burning sulphur, which modifies the sulphur components already in the hops but which is said to stabilize the aroma and colour.
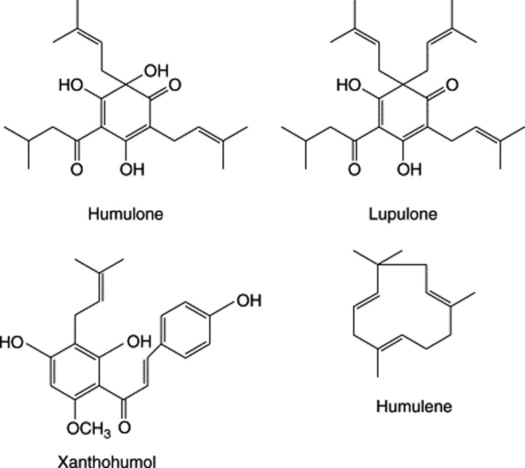
The bracts and stipules of the hop contain tannin but the odour and taste of the drug are mainly due to the very complex secretion contained in the lupulin glands. On distillation the fruits yield 0.30–1.0% of an oil composed of well over 100 components and containing terpenes, sesquiterpenes including humulene (Fig. 21.3) and compounds such as 2-methyl-but-3-ene-2-ol and 3-methylbutanoic acid. The two latter, and related substances, increase significantly during processing of the fresh hops. The bitterness is due to crystalline phloroglucinol derivatives known as α-acids (e.g. humulone), β-acids (e.g. lupulone) and also about 10% of resins. 2,3,4-Trithiapentane, S-methylthio-2-methylbutanoate, S-methylthio-4-methyl-pentanoate and 4,5-epithiocaryophyllene have been isolated from the volatile oil of unsulphurated hops.
Male fern.
VANILLA AND VANILLIN
Constituents
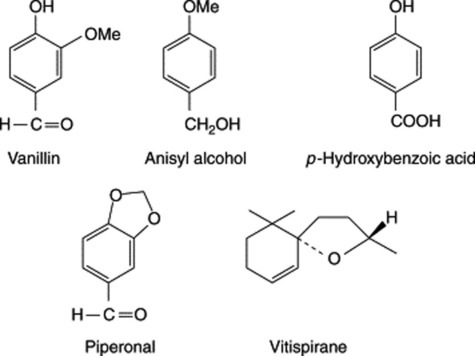
Green vanilla contains glycosides, namely glucovanillin (vanilloside) and glucovanillic alcohol. During the curing these are acted upon by an oxidizing and a hydrolysing enzyme which occur in all parts of the plant. Glucovanillic alcohol yields on hydrolysis glucose and vanillic alcohol; the latter compound is then by oxidation converted into vanillic aldehyde (vanillin). Glucovanillin, as its name implies, yields on hydrolysis glucose and vanillin (Fig. 21.4).
Vanillin BP/EP
Vanillin BP is the aldehyde corresponding to methyl-protocatechuic acid and has been synthesized in a number of ways. Large quantities of it are prepared from eugenol isolated from oil of cloves (q.v.) or from guaiacol (methyl catechol). It can also be produced by microbial oxidation of eugenol. In the plant glucovanillin is biosynthesized via ferulic acid (see Fig. 21.2). Synthesis begins when elongation of the fruit ceases, which is about 8 months after pollination; before this, other phenolic glycosides predominate.
BEARBERRY LEAVES
Microscopical features include: an upper epidermis of polygonal cells with a thick cuticle; lower epidermis with anomocytic stomata and surrounded by 5–11 subsidiary cells; scars of trichome bases, occasional conical trichomes, crystal fibres.
Bearberry contains the glycosides arbutin (Table 21.1) and methylarbutin, about 6–7% of tannin, (+)-catechol, ursone and the flavone derivative quercetin. Some 14 phenolic acid constituents, including gallic and ellagic acids, have been recorded.
CAPSICUM
Constituents
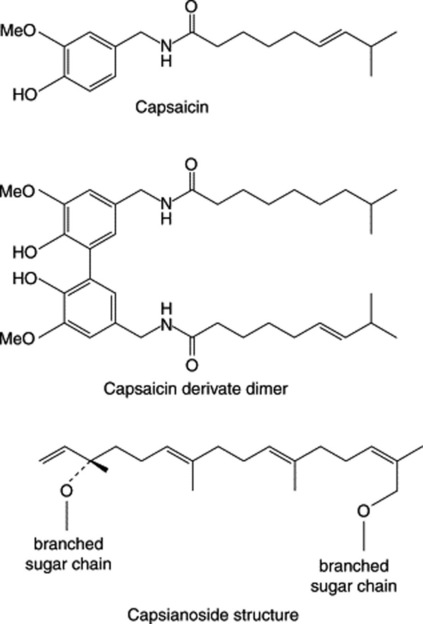
In a study of the water-soluble constituents of the fruits of three varieties of C. annuum, Izumitani et al. (Chem. Pharm. Bull., 1990, 38, 1299) isolated twelve novel acyclic glycosides (geranyllinalool derivatives) named capsianosides A–F (dimeric esters of acyclic diterpene glycosides) and capsianosides I–V (monomeric compounds of acyclic diterpene glycosides). Further capsianosides have now been reported by J.-H. Lee et al., (Chem. Pharm. Bull., 2006, 54, 1365). T. Ochi et al., (J. Nat. Prod., 2003, 66, 1094) record a dimeric capsaicin having almost the same antioxidant activity as capsaicin but with no pungent taste (Fig. 21.5).
Biogenesis of capsaicin
Work by Leete and Louden on C. frutescens and by Bennett and Kirby on C. annuum demonstrated that phenylalanine is incorporated into the C6–C1 vanillyl unit of capsaicin, the C-3 of phenylalanine giving the methylene group of the vanillylamine residues; the incorporation probably proceeds via cinnamic, p-coumaric, caffeic and protocatechuic acids. Tyrosine did not appear to be a probable precursor. Leete’s feeding experiments with [U-14C]-valine gave incorporations consistent with the hypothesis that the C10 isodecanoic acid is formed from isobutyryl coenzyme A and three acetate units. More recent work showed that the homo derivatives (C11 acid) are formed from leucine and isoleucine.
Tests and standards
The official TLC test for identity establishes the presence of capsaicin and dihydrocapsaicin in the sample. The synthetic equivalent of capsaicin, nonivamide (pelargonyl vanillylamide), a commercial product used as a flavour in the food industry and in medicine as a topical rubifacient, is limited by a liquid chromatographic assay to a maximum of 5% of the total capsaicinoid content. Liquid chromatography is also used to determine the total capsaicinoid content (minimum 0.4%). A number of colorimetric assays can be used for the quantitative determination of capsaicin (see Table 16.5); the BPC 1973 utilized ultraviolet absorption at 248 and 296 nm for the ointment and oleoresin. Foreign matter should not exceed a maximum of 2%; fruits of C. annuum L. var. longum (Sendtn.) (see ‘Bombay capsicums’ below) should be absent.
TANNINS
The above tannin-protein co-precipitation is important not only in the leather industry but also in relation to the physiological activity of herbal medicines, taste of foodstuffs and beverages, and in the nutritional value of feeds for herbivores. Environmental factors affecting this process have been studied by H. Kawamoto and F. Nakatsubo (Phytochemistry, 1997, 46, 479).
Hydrolysable tannins
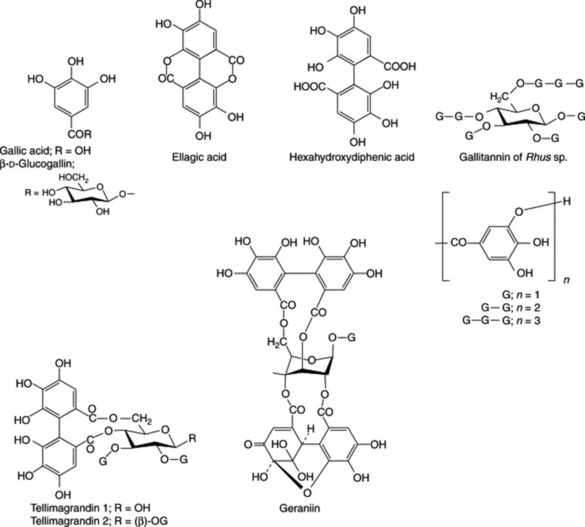
These may be hydrolysed by acids or enzymes such as tannase. They are formed from several molecules of phenolic acids such as gallic and hexahydroxydiphenic acids which are united by ester linkages to a central glucose molecule. A simple tannin illustrating this point is one derived from a species of sumac (Rhus), with a possible structure as shown in Fig. 21.6. Like gallic acid their solutions turn blue with iron salts. They were formerly known as pyrogallol tannins, because on dry distillation gallic acid and similar components are converted into pyrogallol. Two principal types of hydrolysable tannins are gallitannins and ellagitannins which are, respectively, composed of gallic acid and hexahydroxy-diphenic acid units. Ellagic acid (the depside of gallic acid) can arise by lactonization of hexahydroxydiphenic acid during chemical hydrolysis of the tannin; thus, the term ellagitannin is a misnomer.
Ellagitannins found in plants of medicinal interest, and for which structures have been elucidated include geraniin (Herb Robert and American cranesbill) and tellimagrandins 1 and 2 (Oak bark, Pomegranate and Meadowsweet); Fig. 21.6.
For an article on the classification of oligomeric hydrolysable tannins and the specificity of their occurrence in plants see Okuda et al., Phytochemistry, 1993, 32, 507.
Examples of drugs containing hydrolysable tannins are:
Condensed tannins (proanthocyanidins)
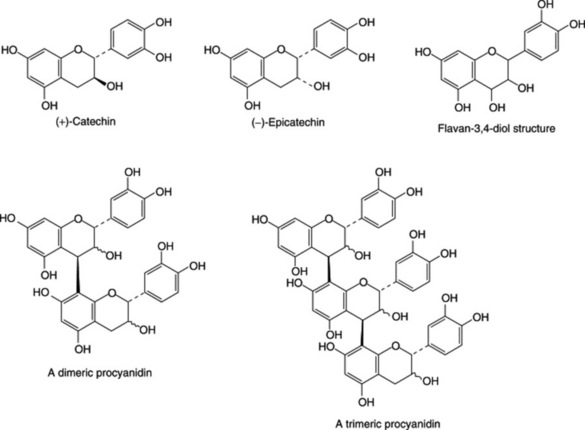
Unlike hydrolysable tannins, these are not readily hydrolysed to simpler molecules and they do not contain a sugar moiety. They are related to the flavonoid pigments and have polymeric flavan-3-ol structures. Catechins, which also occur with the tannins and flavan-3,4-diols (leucoanthocyanidins) are intermediates in the biosynthesis of the polymeric molecules. Stereochemical variations add to the variety of possible structures. Monomeric, dimeric and trimeric forms are illustrated in Fig. 21.7. Work by Japanese phytochemists has exploited modern techniques for separating and determining the structures of these oligomers and polymers including those of cassia bark, Cassia fistula, cinchona, Quercus and rhubarb.
‘Complex tannins’
This term has been applied by Okuda to a newly-discovered group of tannins which are biosynthesized from both a hydrolysable tannin (mostly a C-glucoside ellagitannin) and a condensed tannin. The union occurs through a C–C bond between the C-1 of the glucose unit of the ellagitannin and the C-8 or C-6 of the flavan-3-ol derivative. The monomers are also involved in oligomer formation.
Pseudotannins
Properties and tests
Medicinal and biological properties
Tannin-containing drugs will precipitate protein and have been used traditionally as stypics and internally for the protection of inflamed surfaces of mouth and throat. They act as antidiarrhoeals and have been employed as antidotes in poisoning by heavy metals, alkaloids and glycosides. In Western medicine their use declined after World War II when it was found that absorbed tannic acid can cause severe central necrosis of the liver. Recent studies have concentrated on the antitumour activity of tannins (M. Ken-ichi et al., Biol. Pharm. Bull., 1993, 16, 379) and it has been shown that, to exhibit a strong activity, ellagitannin monomer units having galloyl groups at the O-2 and O-3 positions on the glucose core(s), as in the tellimagrandins (Fig. 21.6) are required. Anti-HIV activity has also been demonstrated.
OAK BARK
The commercial bark, obtained principally from E. and S.E. Europe, occurs as channelled pieces, 3–4 mm thick and of various lengths. Younger, thinner pieces have a smooth, greyish-green cork with lenticels, older pieces have a greyish-brown rhytidome and show a fracture, granular in the outer part and fibrous and splintery in the inner part. Conspicuous features of the reddish-brown powder include cork cells, lignified fibres with crystal sheaths of calcium oxalate, pitted sclereids and cluster crystals of calcium oxalate in parenchymatous cells.
Oak bark is used medicinally for its astringent properties and industrially for tanning and dyeing.
HAMAMELIS LEAF
Microscopical characters
The drug has very distinctive microscopical characters. These include characteristic stomata present on the lower surface only; very large lignified idioblasts, crystal cells accompanying the pericyclic fibres, tannin-containing cells and, especially in young leaves, stellate hairs. The calcium oxalate is in monoclinic prisms 10–35 μm long. The stellate hairs (Fig. 42.1H) consist of 4–12 cells united at the base. Each cell is thick-walled and up to 500 μm long.
Constituents
Hamamelis contains gallitannins, ellagitannins, free gallic acid, proanthocyanidins, bitter principles and traces of volatile oil. With ferric chloride solution the gallitannins and the free gallic acid give a blue colour and the ellagitannins, green.
HAWTHORN
Characters
Characteristic of a number of genera of the family Rosaceae, so-called hawthorn berries are false fruits (pomes, and not in the strict botanical sense berries) in which the carpels become adherent to the hollow, fleshy receptacle and the sepals, petals and stamens become situated at the upper end of the fruit. The carpels become stony so that the pome comes rather to resemble a drupe (Ch. 41). The false fruits of C. monogyna with one carpel contain a single stony true fruit whereas those of C. laevigata with two or three carpels contain two or three fruits.
The dried reddish-brown to dark red fruits have a slight odour and mucilaginous, slightly acid taste; with C. monogyna they are up to 10 mm in length and slightly larger for C. laevigata. At the upper end of the false fruit are the remains of the five reflexed sepals which surround a shallow depression from the base of which arise stiff lignified tufts of trichomes and the remains of the style (two styles with C. laevigata). The base of the fruit may be either attached to a pedicel or show the scar of attachment of the latter.
AGRIMONY
Agrimony BP/EP, BHP family Rosaceae consists of the dried flowering tops of Agrimonia eupatoria L.
RHATANY
Allied species
The roots of several other species are occasionally encountered in commerce, but the Peruvian drug is the only one generally available. Krameria cystisoides of Mexican origin has indigenous medicinal uses. It contains over 20 compounds of the lignan, neolignan and norneolignan type. Similar constituents are reported for K. lanceolata; see H. Achenbach et al., Phytochemistry, 1987, 26, 1159; 1989, 28, 1959.
CATECHU
Chemical tests
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree