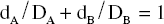Chapter 7 Synergy and other interactions in phytomedicines
MEASURING SYNERGY
Although the idea of synergy is easy to understand, the measurement of it is more problematic. It is fairly straightforward to identify synergy when one of the agents is inactive and a combination of this with an active agent produces an effect greater than that observed for the active alone (although this is more correctly termed potentiation), but difficulties in measurement arise when more than one (and there might easily be several) are active. Various methods for calculation have been devised over the years, but the following are now thought to be the most useful:
PREDICTION OF EFFECTS
Synergy is deemed present if the total effect of a combination is greater than would be predicted on the basis of expected additive effects of the mixture. Such additivity expectations can be derived from dose addition or independent action. Often, anticipated additivity is calculated by simply adding up the effects of individual mixture components, but this method can produce paradoxical and erroneous results and is therefore deemed unreliable (A. Kortenkamp and R. Altenburger, 1998; see legend for Fig. 7.1). The opposite applies for antagonism, which is observed less than would have been predicted.
THE ISOBOLE METHOD
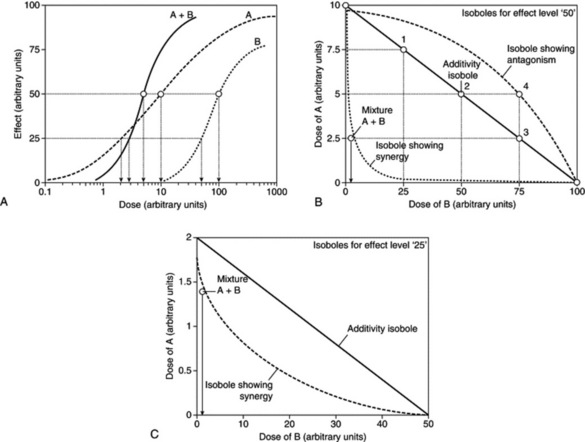
The isobole method is an application of dose addition. It is unequivocal proof of synergy because it is independent of any knowledge of mechanisms and applies under most conditions. It makes no assumptions about the behaviour of each agent and is applicable to multiple components of up to three constituents, so can be applied to the analysis of effects in herbal mixtures. The isobole method uses graphs constructed to show curves (isoboles) describing combinations of two compounds, A and B, which produce the same specified action – which can be any measurable effect (Fig. 7.1A,B). The axes of the graph (Fig. 7.1B) represent doses of the two compounds on a linear scale. A line joining the iso-effective doses A and B of the single agents predicts the combinations of A and B that will yield the same effect, provided the interaction between A and B is only additive. This is the ‘additivity line’, in which case, there is no interaction between the two agents and they could be considered to be behaving like dilutions of each other.
However, if synergy occurs, then smaller amounts are needed to produce the effect (i.e. the effect of the combination exceeds expectation) and the equation becomes dA / DA + dB / DB < 1, and the isobole is said to be ‘concave-up’. The opposite applies for antagonism, and the equation becomes dA / DA + dB/DB > 1, producing a ‘concave-down’ isobole. It is actually possible to have synergy at a particular dose combination with antagonism at a different combination, and this would be reflected in the isobole. The position of isoboles varies depending on the effect level chosen for analysis (see Fig. 7.1B,C).
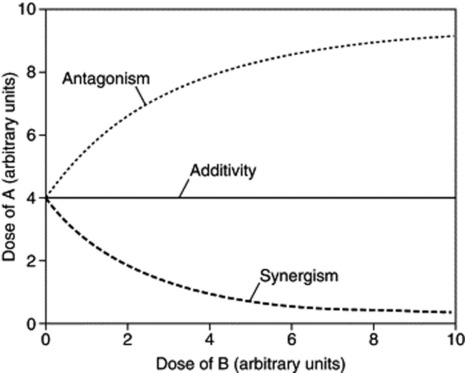
The isobole method can also be applied to mixtures in which only one of the two agents is active; in effect ‘potentiation’. In this case, the iso-effective dose of the agent lacking activity can be regarded as being infinitely large, so the additivity isobole runs parallel to the respective dose axis. Synergism will again yield a concave-up isobole and antagonism a concave-down isobole, as shown in Fig. 7.2.
DEMONSTRATING SYNERGY AND POLYVALENT ACTION IN PHYTOMEDICINES
In conventional medicine it is now common practice to use several drugs to treat a single complaint, such as in hypertension, psychoses and especially cancer, and this approach applies even more to plant extracts, because combinations are already present within the plant. There might be other sound reasons for not isolating individual components in some herbs such as ginkgo, St John’s wort and ginseng, because these are traditionally used as standardized extracts for which there is positive clinical data. In some cases, the active ingredients may not even be fully known, and if synergy is involved, then bioassay-led fractionation (the usual method for identifying actives) would not even be possible (see P. Houghton, Phytother. Res., 2000, 14(6): 419–423). The identities of the main actives of many important herbs are still under discussion, and it would be unwise to exclude, by overpurification, any constituents that might contribute to efficacy. Even if the phytochemistry of a plant is well documented, the actual contribution of individual components to the overall effect might not have been ascertained. Examples include hawthorn (Crataegus oxycantha) as a cardiac tonic, hops (Humulus lupulus) as a sedative, black cohosh (Cimifuga racemosa) and chasteberry (Vitex agnus-castus) as hormone-balancing agents in women, saw palmetto (Serenoa repens) as an antiandrogen for prostatic hyperplasia, and devil’s claw (Harpagophytum procumbens) as an anti-inflammatory agent. In other cases, the actives are unstable, and attempts to remove them from the ‘protection’ of the herb or whole extract could render them inactive. Here the obvious examples are garlic (Allium sativum) and valerian (Valeriana spp.). Garlic is often formulated as a product containing the precursor alliin, and the enzyme alliinase, which in solution (i.e. in the stomach) liberates the active allicin and other unstable, but still active, decomposition products. This is not synergy but, effectively, a drug-delivery system.