Fig. 1
Sharpening sensory acuity through lateral interaction. In the visual system, a center-surround receptive field sharpens the spatial input for visual acuity. By analogy, a stimulus-counter-stimulus receptive field may sharpen the modality input for somatosensory acuity. In this way, counter stimuli could, for example, inhibit itch
Some indirect evidence for this idea came from two groups that were examining the role of vGLUT2 in somatosensation. These groups made the same fundamental observation, which unexpectedly gave insight into the coding of itch (Lagerstrom et al. 2010; Liu et al. 2010). Glutamate is the fast excitatory neurotransmitter that is released by all primary somatosensory neurons. Although there are three glutamate transporters (vGLUT1–3), many sensory neurons express only vGLUT2. Hence, when vGLUT2 is conditionally removed from primary afferents, the “vGLUT2-only” subset is no longer able to signal via glutamate. Since the vGLUT2-only subset is a large proportion of the C fibers, it was not surprising that the loss of vGLUT2 in primary afferents resulted in blunted pain responses. However, the completely unexpected finding was the vGLUT2-lacking mice scratched all the time and showed elevated itch. These findings imply that glutamate signaling from vGLUT2-only primary afferents normally inhibits itch. However, the neural circuitry underlying this phenomenon remained unclear.
Around this time, strong evidence emerged, suggesting that itch is under inhibitory control at the level of the spinal cord. To study the neural circuits underlying itch, Akiyama et al. (2011) had developed a clever method to record from spinal neurons that are presumably involved in the transmission of itch: they used a model of dry skin itch (to drive ongoing itch) and then recorded from spontaneously active neurons in the spinal cord. Consistent with the idea that counter stimuli inhibit itch, they found that scratching, pinch, and noxious heat inhibited the firing rate of spontaneously active neurons. Moreover, treatment with either strychnine (to block glycinergic inhibition) or bicuculline and saclofen (to block GABAergic inhibition) strongly reduced the scratch-evoked inhibition of these cells. These findings suggested that both GABAergic and glycinergic inhibitory mechanisms in the spinal cord are involved in the inhibition of itch. But the identity of these inhibitory interneurons was not known.
Now, recent work from our lab has revealed that itch is inhibited by a population of spinal interneurons called B5-I neurons, and there is some tantalizing evidence that B5-I may mediate the inhibition of itch by counter stimuli (Kardon et al. 2014; Ross et al. 2010). Here, we review how these inhibitory interneurons were first discovered and what we know about them.
2 Transcription Factors and the Development of the Dorsal Spinal Cord
Itch, pain, touch, and temperature are first detected in primary sensory afferents that convey this information to the dorsal horn in the spinal cord, particularly laminae I–III. Notably, less than 1 % of the neurons in the dorsal spinal cord are projection neurons that convey somatosensory input to the brain (Todd 2010). The vast majority of neurons in the dorsal horn (~99 %) are local interneurons, a fact which strongly implicates spinal microcircuits in the integration of somatosensory input. Moreover, it is becoming increasingly clear that these neural networks are made of discrete subtypes of spinal interneurons that form stereotyped connections with one another. Importantly, the wiring among these neurons appears to be developmentally programmed by a series of transcription factors. Thus, to understand these local networks, we need to understand the ontogeny of spinal interneurons.
Interneurons within the dorsal horn arise from progenitors that reside in the ventricular zone of the developing spinal cord (Helms and Johnson 2003). These neurons, which are among the last to differentiate, undergo their final round of cell division in between embryonic day 12 and embryonic day 14.5. Two basic types of neurons are born at this time, the so-called dorsal interneurons late A and B (dILA and dILB), which develop into inhibitory and excitatory neurons of the dorsal horn, respectively. Various transcription factors are expressed in neural progenitors and early postmitotic neurons during this time, and these factors are involved in specifying neuronal identity and mediating the differentiation of a neuronal precursor into a specific cell type with stereotyped connectivity.
In particular, the transcription factors of the basic helix-loop-helix (bHLH) and homeodomain factors appear to play key roles in these processes. For instance, inhibitory interneurons in the dorsal horn are not generated in mice lacking the bHLH factor Ptf1a, emphasizing the important role of the Ptf1a in mediating inhibitory neuronal fate (Glasgow et al. 2005). Excitatory neurons, in contrast, require the homeodomain factor Tlx3, which is required to suppress the GABAergic fate (Cheng et al. 2004). Both excitatory and inhibitory neurons diversify further during maturation into a large array of distinct neural subtypes (the number of which is not yet known). For instance, various neuropeptides, receptors, and other neuronal markers are expressed by distinct subpopulations of dorsal horn neurons (Polgar et al. 2013b). But, while transcription factors are thought to mediate the terminal differentiation of neurons and their connectivity, the identity of these factors and their specific functions remain poorly understood. Bhlhb5 (also called Bhlhe22) is a bHLH transcription factor that is expressed in the dorsal spinal cord within subsets of dILA and dILB neurons from the time they are postmitotic approximately until P14 (Ross et al. 2010). Thus, based on the expression pattern of Bhlhb5, we hypothesized that this transcription factor was involved in the terminal differentiation and connectivity of subsets of neurons in the dorsal horn.
3 Mice Lacking the Transcription Factor Bhlhb5 Show Abnormally Elevated Itch
Bhlhb5 is a neural-specific basic helix-loop-helix (bHLH) transcription factor related to the Drosophila proneural factor atonal (Ross et al. 2003). Bhlhb5 and other closely related family members (namely, Bhlhb4 and Oligs1–3) all function as transcriptional repressors. However, Bhlhb5 is distinct from its family members because Bhlhb5 is selectively expressed in postmitotic neurons rather than in proliferating progenitors. Thus, whereas the Oligs (and likely Bhlhb4) are involved in neuronal fate specification, Bhlhb5 is involved in terminal neuronal differentiation. To investigate the function of Bhlhb5, two independent groups—ours and that of Lin Gan—made Bhlhb5 knockout mice. These studies revealed an important role for Bhlhb5 in the retina, where it is required for the survival of some amacrine and cone bipolar cells (Feng et al. 2006). In addition, Bhlhb5 is required for the acquisition of area-specific fates in the cortex (Joshi et al. 2008). We found that Bhlhb5 is a transcriptional repressor that uses Prdm8 as an obligate cofactor and that both factors are required for the proper axonal targeting of all cortical projection neurons (Ross et al. 2012). Thus, Bhlhb5 has multiple roles in different regions of the nervous system. However, the most striking phenotype of Bhlhb5 −/− mice is that they all develop self-inflicted skin lesions, which prompted us to investigate somatosensation in these mice (Ross et al. 2010).
Initially, it was not clear why Bhlhb5 −/− mice, which behave normally until around 4–6 weeks of age, suddenly develop self-inflicted skin lesions. At the time of these studies, only a few animals with skin lesions had been analyzed in detail, and in these cases it was concluded that the mice in question either lacked sensitivity to pain (Drg11 −/− mice) or suffered from obsessive-compulsive disorder (Hoxb8 −/− mice) (Chen et al. 2001; Greer and Capecchi 2002). Indeed, when we (naively) first analyzed somatosensation in adult Bhlhb5 −/− mice (which, importantly, already had skin lesions), we found that these mice showed a very blunted response to noxious input. Based on these findings, we erroneously concluded that the self-injury observed in Bhlhb5 −/− mice was due to an absence of pain. Fortunately (and as a testimony to the efficacy of the review process), the reviewers of our manuscript questioned our interpretation and asked us to investigate the possibility of abnormal itch in Bhlhb5 −/− mice. When we reevaluated the behavior of Bhlhb5 −/− mice, this time analyzing mice before the onset of skin lesions, we realized that our first interpretation was completely wrongheaded. In fact, prior to the onset of skin lesions, Bhlhb5 −/− mice show normal responses in most sensory tests including chemical, mechanical, and heat nociception (Ross et al. 2010). However, Bhlhb5 −/− mice scratch significantly more than wild-type littermates following the application of all of the itch-inducing agents tested. Therefore, the lack of Bhlhb5 expression led to an increase in itch sensitivity, but left pain and other somatosensory modalities relatively intact. Furthermore, these findings suggested that the self-inflicted skin lesions in Bhlhb5 −/− mice were the result of excessive licking and scratching due to elevated itch.
4 Pathological Itch in Bhlhb5 −/− Mice Is Due to the Loss of B5-I Neurons
Having determined that Bhlhb5 −/− mice have abnormally elevated itch, the next step was to identify which cells were responsible for this phenotype. Since Bhlhb5 is expressed in numerous regions of the nervous system, the cellular basis of elevated itch was not completely obvious. Though Bhlhb5 is expressed in subsets of neurons in the dorsal horn (where itch is first integrated), this transcription factor is also expressed in some primary sensory afferents, the brainstem, and many other regions of the brain that might theoretically be involved in the processing of itch. To determine the neurons responsible for the elevated itch in Bhlhb5 −/− mice, we used a genetic approach to selectively remove Bhlhb5 from different regions of the nervous system (Ross et al. 2010). Using this conditional ablation strategy, we were able to ask whether deletion of Bhlhb5 in specific areas of the nervous system was sufficient to recapitulate the phenotype seen in the constitutive Bhlhb5 −/− mice. Upon loss of Bhlhb5 from primary afferents, the resulting mice were normal with respect to itch. Likewise, upon loss of Bhlhb5 from the dorsal telencephalon, the resulting mice had no sensory phenotypes. However, loss of Bhlhb5 from the spinal cord (using the Hoxb8-cre line) was sufficient for the abnormally elevated itch and the development of skin lesions (unpublished observation). This finding suggested a key role of Bhlhb5 in the spinal cord. Since Bhlhb5 is expressed in both excitatory and inhibitory neurons within the spinal cord, we used cre lines that caused selective removal of Bhlhb5 in excitatory and inhibitory neurons, respectively, to determine which type of spinal neurons were involved. These experiments revealed that loss of Bhlhb5 within excitatory neurons of the dorsal horn (using Tlx3-cre) had no effect on itch sensitivity, whereas loss of Bhlhb5 within inhibitory neurons (using Pax2-cre) was sufficient for abnormally elevated itch. Together, these experiments revealed that Bhlhb5 is required in inhibitory spinal interneurons for normal itch.
In newborn mice, Bhlhb5 is expressed in 7 % of neurons within the dorsal spinal cord. Of these, approximately one quarter of Bhlhb5-expressing neurons are excitatory and three quarters are inhibitory. At the time, there were no other markers for the Bhlhb5-expressing cells, and so there was no way to see what happened to the Bhlhb5-expressing cells in the absence of Bhlhb5. To resolve this problem, we used another genetic approach to permanently label all the cells that had ever expressed Bhlhb5. Specifically, we generated a Bhlhb5-cre knockin allele, which we then crossed with cre-responsive reporters (Ross et al. 2010). Using this approach, we discovered that Bhlhb5 is required for the survival of Bhlhb5-expressing neurons in the spinal cord. Without it, many of the neurons that should have expressed Bhlhb5 were missing. This discovery implied that the loss of a specific population of inhibitory interneurons during development results in abnormal itch, and we called these spinal interneurons B5-I neurons, since they are the Inhibitory subset of Bhlhb5-expressing neurons.
5 B5-I Neurons Function to Inhibit Itch
Although we had identified the neurons responsible for abnormal itch, we still didn’t know very much about them. To study these cells in greater detail, we began collaborating with Andrew Todd. His previous work had revealed that spinal inhibitory neurons can be divided into subgroups based on the expression of neurochemical markers (Polgar et al. 2013b). In particular, approximately one half of the inhibitory neurons in laminae I and II of the spinal cord express somatostatin receptor sst2A, and B5-I neurons were found to belong to this subset (Kardon et al. 2014). The discovery that all B5-I neurons express sst2A (which inhibits neurons) was important because it provided us with the tools that we needed to address a key question. Specifically, hitherto it was still not clear whether B5-I neurons function in the adult animal to inhibit itch or whether the survival of B5-I neurons was critical for the establishment of proper itch circuits—in other words, we could not distinguish between an adult function and a developmental role for B5-I neurons. Fortuitously, the finding that B5-I neurons express sst2A provided an opportunity to directly test whether B5-I neurons inhibit itch in adult mice. Specifically, we reasoned that if B5-I neurons suppress itch, then inhibition of B5-I neurons through activation of sst2A would result in spontaneous itch. In keeping with this idea, we found that intrathecal injection of the sst2A agonist, octreotide, resulted in spontaneous scratching behavior. Moreover, this effect was lost in Bhlhb5 −/− mice that are lacking B5-I neurons (Fig. 2). These data revealed that disinhibition of B5-I neurons causes itch, indicating that B5-I neurons normally function to inhibit itch.
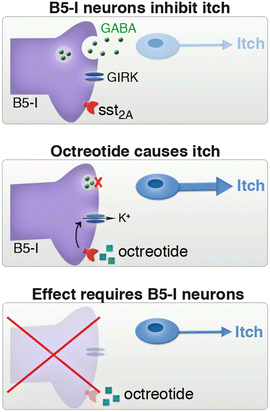

Fig. 2
Evidence that B5-I neurons function to inhibit itch. (a) Normally, itch is inhibited by B5-I neurons. (b) Activation of the somatostatin receptor sst2A with octreotide results in the inhibition of B5-I neurons and spontaneous itch. (c) Octreotide has no effect in Bhlhb5−/− mice, which are lacking B5-I neurons
6 B5-I Neurons Release the Kappa Opioid Dynorphin and Kappa Agonists Inhibit Itch
The inhibitory interneurons that express sst2A are not a single population, and previous work by Andrew Todd had shown these interneurons can be further subdivided based on the expression of distinct neurochemical markers (Polgar et al. 2013a; Spike et al. 1998). When we looked at which of these subpopulations made up B5-I neurons, we discovered that B5-I neurons are composed of two mostly nonoverlapping subpopulations, one expressing the neuropeptide galanin and one expressing neuronal nitric oxide synthase (nNOS). Moreover, both galanin-expressing and nNOS-expressing subpopulations were almost completely missing in Bhlhb5 −/− mice, whereas other populations of inhibitory neurons were unaltered (Kardon et al. 2014).
This discovery raised the important question of whether one or both of these subpopulations are involved in the inhibition of itch. Although we still don’t know the answer to this question with certainty, we were particularly interested in the galanin subpopulation because previous work had shown that galanin-expressing interneurons also express the endogenous kappa opioid, dynorphin (Sardella et al. 2011). The idea that the release of dynorphin from B5-I neurons could be a potential mechanism through which they inhibit itch sensation was an attractive idea since there was already some precedent for the idea that kappa opioids inhibit itch (Inan and Cowan 2004; Ko et al. 2003; Togashi et al. 2002). Consistent with these previous studies, we found that pretreatment with kappa opioid agonists significantly decreased scratching in response to both histamine-dependent and histamine-independent pruritogens in wild-type mice (Kardon et al. 2014). Kappa opioid agonists also significantly reduced the elevated scratching response observed in Bhlhb5 −/− mice, consistent with the idea that it is the absence of dynorphin in Bhlhb5 −/− mice that is partially responsible for their elevated itch.
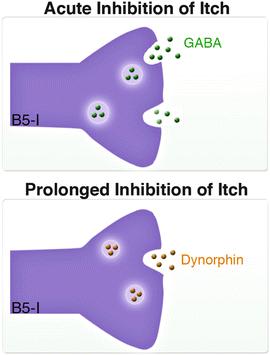
These findings raised the questions as to whether the GABA/glycine-mediated inhibition provided by B5-I galanin and nNOS cells was responsible for the elevated itch in Bhlhb5 −/− mice or whether it was due solely to the decrease of dynorphin signaling. To evaluate this question, we analyzed the preprodynorphin knockout mice (PPD −/− ), which are missing dynorphin but not spinal dynorphin-expressing neurons. We found that loss of PPD −/− had no effect on itch sensitivity, and PPD −/− mice do not develop self-inflicted skin lesions (Kardon et al. 2014). These observations point to a key difference between the loss of a neuropeptide and a loss of a neuronal subtype. Thus, whereas compensatory mechanisms may be able to atone for the loss of dynorphin, they cannot fully compensate for the loss of dynorphin-releasing neurons in Bhlhb5 −/− mice. In conjunction, this evidence points to a role for both GABA/glycine- and kappa opioid-mediated inhibition in the regulation of itch sensation. One possibility is that GABA and glycine, which are fast-acting neurotransmitters, are involved in the immediate relief of itch that is felt upon scratching, whereas dynorphin, which is a neuromodulator that signals via Gi/o-coupled signaling cascades, is involved in the prolonged inhibition of itch (Fig. 3).