Fig. 1
Lack of corresponding functionally defined C-fiber classes in primates and molecular markers in rodents. Only for the markers of Mas-related G-protein-coupled receptor B4 (MrgprB4) and vesicular glutamate transporter (VGLUT3) has the link to the low-threshold C-fibers (“C-touch”) been established. Yet there is no link of identified markers of pruriceptors on the right and electrophysiologically defined C-fiber classes in primates
C-fibers responding to histamine iontophoresis in parallel to the itch ratings of subjects have been discovered among the group of mechano-insensitive C-afferents (Schmelz et al. 1997), suggesting that there is a specific pathway for itch. In contrast, the most common type of C-fibers, mechano-heat-responsive nociceptors (CMH or polymodal nociceptors), is either insensitive to histamine or only weakly activated by this stimulus (Schmelz et al. 2003). Hence, this fiber type cannot account for the prolonged itch induced by the iontophoretic application of histamine. Yet, when histamine is injected intracutaneously, polymodal nociceptors are also activated for several minutes (Johanek et al. 2008). Thus, a contribution of this fiber class to histamine-induced itch cannot be entirely ruled out.
The histamine-sensitive pruriceptors among the mechano-insensitive C-nociceptors are characterized by a particularly low conduction velocity, large innervation territories, mechanical unresponsiveness, and high transcutaneous electrical thresholds (Schmelz et al. 1997, 2003; Schmidt et al. 2002). In line with the large innervation territories of these fibers, two-point discrimination for histamine-induced itch is poor (15 cm in the upper arm) (Wahlgren and Ekblom 1996). The excellent locognosia for histamine-induced itch in the hand (Koltzenburg et al. 1993) might therefore be based on central processing compensating for the low spatial resolution in the periphery.
The relative prevalence of the different C-fiber types in human skin nerves has been estimated from recordings in the superficial peroneal nerve (Schmidt et al. 1997). Polymodal nociceptors, which respond to mechanical, heat, and chemical stimuli, are about four times as abundant as the mechano-insensitive nociceptors in young healthy volunteers, but their proportion decreases in the elderly (2.5 times) (Namer et al. 2009). Mechano-insensitive nociceptors (Schmidt et al. 1995) are activated by chemical stimuli (Schmelz et al. 2000b) and can be sensitized to mechanical stimulation in the presence of inflammation (Schmidt et al. 1995; Schmelz et al. 2000b). Among the mechano-insensitive afferent C-fibers, only a subset of units shows a strong and sustained response to histamine. They comprise about 20 % of the mechano-insensitive class of C-fibers, i.e., about 5 % of all C-fibers in the superficial peroneal nerve. Specific activation of histamine-positive chemonociceptors by PgE2 (Schmelz et al. 2003) in combination with the pruritogenic effects of prostaglandins (Neisius et al. 2002) provides a strong argument for a specific neuronal system for the itch sensation which is separate from the pain pathway.
The axon reflex flare is a neurogenic vasodilation that characteristically surrounds a histamine stimulation site; it is induced by neuropeptide release from mechano-insensitive C-fibers (Schmelz et al. 2000a). The absence of an axon reflex flare therefore suggests that the itch is independent of histamine-sensitive C-fibers. Indeed, itch was induced by papain in an early study in the absence of a flare response indicating a histamine-independent action (Hägermark 1973). Itch without axon reflex flare can also be elicited by weak electrical stimulation (Shelley and Arthur 1957; Ikoma et al. 2005), providing further evidence that the sensation of itch can be dissociated from cutaneous vasodilation.
Cowhage spicules inserted into human skin produce itch in an intensity which is comparable to that following histamine application (LaMotte et al. 2009; Sikand et al. 2009). However, mechano-heat-responsive “polymodal” C-fiber afferents, the most common type of afferent C-fibers in the human skin (Schmidt et al. 1995), can be activated by cowhage in the cat (Tuckett and Wei 1987) and, according to recent studies, also in nonhuman primates (Johanek et al. 2007, 2008) and in human volunteers (Namer et al. 2008) (Fig. 2).
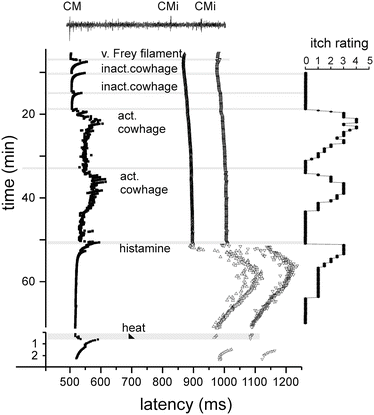

Fig. 2
Specimen of a multifiber recording from a mechano-responsive (CM) and two mechano-insensitive nociceptors (CMi) in human (raw signal with marked action potential on top). Conduction latencies of these three marked fibers (filled square, open triangles) in response to successive electrical stimulation at the receptive field are plotted from top to bottom. When activated by mechanical (v. Frey filament, inactivated cowhage spicules), chemical (active cowhage, histamine), or heat test stimuli (black triangle), C-fibers exhibit an activity-dependent increase of response latency followed by a gradual normalization (“marking”). The mechano-responsive fiber is activated during mechanical stimulation with the v. Frey filament and during application of inactive cowhage, but lasting activation is only seen after application of active cowhage. In contrast, the mechano-insensitive fibers do not respond to cowhage stimulation, but are activated following histamine iontophoresis.
At the right side of the panel, the itch ratings of the subject are depicted which were assessed during this experiment. Ratings are given on a numerical rating scale from 0 (0 = no itch) to 10 (10 = maximal imaginable itch). Inactive cowhage does not evoke any itch, whereas active cowhage and histamine evoke itch of similar time course and intensity, mirroring nicely the activation pattern of the fibers. Modified from Namer et al. (2008)
These fibers are unresponsive to histamine and not involved in sustained axon reflex flare reactions (Schmelz et al. 2000b). This is consistent with the observation that cowhage-induced itch is not accompanied by a widespread axon reflex flare (Shelley and Arthur 1955, 1957; Johanek et al. 2007). While in humans the segregation between histamine-positive mechano-insensitive fibers and cowhage-positive mechanosensitive fibers is clear-cut, in monkeys mechanosensitive C-fibers also responded to histamine (Johanek et al. 2008). The different histamine responses might be explained by higher histamine concentrations upon intradermal injection vs. iontophoresis.
Aδ fibers responding to cowhage insertion for several minutes (Ringkamp et al. 2011) suggest an additional role of afferent input from myelinated fibers. Differential block of myelinated afferents does not reduce capsaicin-induced pain and only slightly reduces histamine-induced itch; however, it massively reduces cowhage-induced itch at least in some of the subjects (Ringkamp et al. 2011). The exact role of Aδ fiber input for the cowhage-induced itch is unclear as reduced skin temperature induced by the nerve blocking maneuver in these experiments might also have reduced cowhage-induced activation.
The active compound, the cysteine protease mucunain, has been identified lately and shown to activate proteinase-activated receptor 2 (PAR 2) and even more potently PAR 4 (Reddy et al. 2008). Given that cowhage spicules can activate a large proportion of polymodal nociceptors, we face a major problem to explain why activation of these fibers by heat or by scratching actually inhibits itch, whereas activation by cowhage produces it. On the other hand, data from monkeys suggest that mechano-heat-sensitive C-nociceptors with a fast response to heating (“QC”) might play a more important role in mediating cowhage-induced itch (Johanek et al. 2008). One might therefore still hypothesize that there is a certain selectivity among mechano-heat-sensitive C-nociceptors for cowhage that would allow the central nervous system to separate nociceptive from pruriceptive stimuli (LaMotte et al. 2014).
2.2 Structural Markers for Classification of Primary Afferents
A variety of marker proteins on sensory afferents are currently used to separate classes of primary afferent sensory neurons in rodents. These markers include sensory transduction proteins such as vanilloid receptors (TRPV1, TRPA1) and purinergic receptors (P2X3), neuropeptides such as substance P and calcitonin gene-related peptide (CGRP), receptors for growth factors such as nerve growth factor (NGF) and glia-derived neurotrophic factor (GDNF), receptors of unknown function such as the family of Mas-related G-protein-coupled receptors (Mrgpr), but also certain staining characteristics such as lectin binding (IB4) (see Fig. 1; right column). Among these markers, there are some with particular relevance for neurons involved in the itch sensation (Akiyama and Carstens 2013). These include histamine H1 receptors, the neuropeptides gastrin-releasing peptide and B-type natriuretic peptide, and the several members of the Mrgpr family (A3, D, C11). Unfortunately, there are only a few examples for a convincing link between the rodent marker and functional neuronal class in primates (see Fig. 1). For a very special subtype of afferent C-fiber, the very low threshold, the so-called C-touch fibers (CT afferents) (Ackerley et al. 2014), links to the expression of MrgprB4 (Vrontou et al. 2013) and to the expression of the glutamate transporter VGLUT3 (Seal et al. 2009) have been described.
In the realm of itch processing, however, we do not have such convincing ties between molecular markers used in rodents and fiber classes in the primate. There is evidence that cowhage induces itch via the activation of proteinase-activated receptors (Reddy et al. 2008). Thus, the activation of QC-type mechano-heat-sensitive nociceptors by cowhage (Johanek et al. 2008) might be a possible link to MrgprC11 (Akiyama and Carstens 2013). Beta-alanine, the activator of MrgprD, does provoke itch in humans (Han et al. 2012; Liu et al. 2012; Qu et al. 2014), but the responsible fiber class is unknown. This is similarly true for BAM8-22, the activator of MrgprC11, which also provokes histamine-independent itch in humans (Sikand et al. 2011) probably via activating MrgprX1, the human homologue of rodent MrgprC11.
The neuropeptides, B-type natriuretic peptide and gastrin-releasing peptide, have both been suggested to represent an itch-specific cotransmitter of the primary afferent pruriceptive neuron (Sun et al. 2009; Mishra and Hoon 2013). Regardless of the current debate (Liu et al. 2014) about the exact role of these two peptides, both will prove highly important to study function/structure relationships by identifying the neurons being involved in itch. On the other hand, therapeutic implications might not be particularly strong as both gastrin-releasing peptide (Sakamoto 2011) and B-type natriuretic peptide (Zhang et al. 2010) have also functions beyond itch processing.
Although we have increased our knowledge on itch-specific targets enormously over the last few years, we still did not succeed to translate the targets and markers from rodents. This is a highly complex task as the traditional markers such as peptidergic (NGF-dependent) vs. non-peptidergic (GDNF-dependent, IB4-positive) nociceptors work fine in the mouse, but do not completely translate into rat or primate. Ultimately, we have to take into account that the crucial functional unit of nociception or pruriception is not a marker molecule, but the cell (Reichling et al. 2013) with its particular peripheral receptors and central connections. Thus, based on the broad knowledge provided by basic research in rodents, we need to translate mediators, markers, and neuronal classes into humans and thereby identify the crucial targets to treat chronic itch in patients.
2.3 Spatial Specificity for Itch?
The spatial characteristics of an itching stimulus need to be considered as it may functionally convert an algogenic mediator into a pruritic mediator: capsaicin, when injected into the skin, is painful, but when applied very locally on a cowhage spicule into the epidermis, it causes itch (Sikand et al. 2009). The highly localized stimulation in the epidermis strongly activates some of the local nociceptors, while their immediate neighbors remain silent, resulting in a mismatch signal of activation and absence of activation from this site. It has thus been hypothesized that this mismatch might be perceived by the central nervous system as itch (Namer et al. 2008). Teleologically, it is obvious that scratching behavior in the case of a highly localized superficial noxious focus is an adequate response as it can eliminate the presumed cause. Moreover, scratching activates all the mechanosensitive nociceptors in the stimulated area, and thus, the mismatch signal of activated and nonactivated nociceptors at this site is terminated. Therefore, it needs to be pointed out that pruritus cannot only be explained by itch-specific or itch-selective neurons (LaMotte et al. 2014) along the specificity theory. In addition, the pure spatial pattern of activated nociceptors might similarly underlie the itch sensation without any requirement of itch-specific primary afferent neurons (Fig. 3).
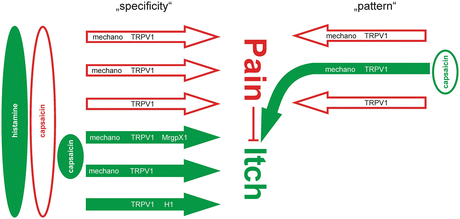

Fig. 3
Specificity versus pattern theory of itch: assuming specific nociceptive primary afferents (open arrows) and specific pruriceptive afferent fibers (filled arrows), one could easily explain itch induced by pruritogens such as histamine by activation of the specific histamine-sensitive pathway. Capsaicin would activate most of the specific nociceptors and pruriceptors and would cause pain according to the spinal inhibition of itch by pain. Very localized application of capsaicin might preferentially activate pruriceptors and thus could provoke itch. Alternatively, localized stimulation with capsaicin activates only a small number of nociceptors (right column: “pattern”). The pattern of activated and nonactivated nociceptors from a given skin site would be interpreted as itch at a spinal level
It is interesting to note that “specificity” is not only discussed for the neurons but also for mediators (Ross 2011). Capsaicin, the classic algogen, generally provokes pain when applied to the human skin. However, it induces itch when applied on the tip of an inactivated cowhage spicule (Sikand et al. 2009). Thus, the response of neurons to the algogen capsaicin might pose an argument against their specificity for itch. Alternatively, the itch evoked by capsaicin applied via a cowhage spicule might be an argument against the nociceptive specificity of capsaicin.
3 Spinal Processing of Itch
The concept of dedicated pruriceptive neurons has been extended by the results obtained from cat spinal cord recordings. A specific class of dorsal horn neurons projecting to the thalamus, which responds strongly to histamine administered to the skin by iontophoresis, has been demonstrated (Andrew and Craig 2001). The time course of these responses was similar to that of itch in humans and matched the responses of the peripheral C-itch fibers. These units were also unresponsive to mechanical stimulation and differed from the histamine-insensitive nociceptive units in lamina I of the spinal cord. In addition, their axons had a lower conduction velocity and anatomically distinct projections to the thalamus. The itch-selective units in lamina I of the spinal cord form a distinct pathway projecting to the posterior part of the ventromedial thalamic nucleus (VMpo) which projects to the dorsal insular cortex (Craig 2002), a region which has been shown to be involved in a variety of interoceptive modalities like thermoception, visceral sensations, thirst, and hunger.
Thus, the combination of dedicated peripheral and central neurons with a unique response pattern to pruritogenic mediators and anatomically distinct projections to the thalamus provides the basis for a specific neuronal pathway for itch.
This is also supported by studies performed with rodents. Dorsal horn neurons bearing the receptor for gastrin-releasing peptide (GRPR) have been identified as being crucial for the scratch behavior in a variety of itch models. There was some reduction of scratching by constitutively inactivating the gene encoding the GRP-receptor gene or pharmacologically blocking the receptor (Sun and Chen 2007). However, the selective deletion of the GRPR-bearing cells by a toxin linked to the GRPR ligand bombesin (bombesin-saporin) completely abolished the scratching behavior, whereas the nociceptive behavior was virtually unchanged (Sun et al. 2009). This indicates that GRPR-expressing dorsal horn neurons may be indispensable for the itch response in this species, though not necessarily only the GRPR receptor alone. However, data on bombesin-induced itch that could not be blocked via a GRPR agonist have shed some doubts on the GRPR specificity of the bombesin results (Su and Ko 2011). Moreover, the B-type natriuretic peptide has been suggested to be the cotransmitter of the primary afferent pruriceptors with GRP being the transmitter of secondary spinal cord neurons (Mishra and Hoon 2013). The exact role of the two peptides is currently debated (Liu et al. 2014). Another type of spinal interneuron involved in itch processing has recently been found. Inhibitory interneurons dependent on the transcription factor Bhlhb5 were described to be involved in itch processing (Ross et al. 2010). Cell-specific deletion of Bhlhb5 resulted in increased scratching to experimental itch stimuli and spontaneous scratching with skin lesions, whereas pain behavior was grossly unchanged (Ross et al. 2010). These results suggest that Bhlhb5-dependent GABAergic inhibitory interneurons are crucial modulators of scratch behavior.
In contrast to the above evidence for a specific pathway for itch, histamine-sensitive projection neurons in the monkey were found to also respond to mechanical stimuli and to capsaicin (Simone et al. 2004; Davidson et al. 2007). Also, in rodents, overlapping between nociceptive and pruriceptive neuronal responses was found (Akiyama et al. 2009, 2010). This is not necessarily a contradiction to the concept of a “specific pathway.” One has to distinguish between “selectivity” (i.e., only a subgroup of neurons responds to a particular pruritogenic substance) and “membrane specificity” (a subgroup of neurons responds only to a group of pruritogenic agents). “Membrane specificity” is not necessarily required for a “specific” or better “selective” pathway. A “selectivity hypothesis” for itch processing has been discussed before by several authors (McMahon and Koltzenburg 1992; Cevikbas et al. 2010; Davidson and Giesler 2010; Handwerker 2010; Patel and Dong 2010; Ma 2010; Ross 2011).
Interestingly, histamine-induced itch and cowhage-induced itch are not only processed separately in the primary afferent neurons, but the separation is maintained at the spinal level. Spinothalamic projection neurons in the dorsal horn could be separated into a histamine- and cowhage-responsive population without overlap (Davidson et al. 2007). Moreover, the thalamic projections of the two subgroups also differed. The histamine-sensitive pathway can be assumed to be selective for itch albeit the recordings in monkeys; STT neurons also suggest some mechanical and capsaicin sensitivity. In contrast, the cowhage-sensitive pathway may be regarded as unspecific as the activation of mechano-heat-responsive polymodal nociceptors is probably underlying the generation of cowhage-induced itch (Davidson and Giesler 2010).
4 Interactions Between Itch Pathways with Painful and Non-painful Stimuli
The inhibition of itch by painful stimuli has been experimentally demonstrated by the use of various painful thermal, mechanical, and chemical stimuli. Electrical stimulation via an array of pointed electrodes (“cutaneous field stimulation”) has also been successfully used to inhibit histamine-induced itch for several hours in an area around a stimulated site of 20 cm in diameter. The large area of inhibition suggests a central mode of action (Nilsson et al. 1997). Consistent with these results, itch is suppressed inside the secondary zone of capsaicin-induced mechanical hyperalgesia (Brull et al. 1999). This central effect of nociceptor excitation by capsaicin should be clearly distinguished from the neurotoxic effect of higher concentrations of capsaicin which destroy most C-fiber terminals, including fibers that mediate itch (Simone et al. 1998). The latter mechanism, therefore, also abolishes pruritus locally, until the nerve terminals are regenerated.
Not only is itch inhibited by enhanced input of pain stimuli, but vice versa, inhibition of pain processing may reduce its inhibitory effect and thus enhance itch (Atanassoff et al. 1999). This phenomenon is not only particularly relevant to the spinally administered μ-opioid receptor agonists which induce segmental analgesia often combined with segmental pruritus (Andrew et al. 2003) but has also been confirmed in animal experiments (Nojima et al. 2003). Recent result suggests that the analgesic and pruritic effects of μ-opioids might be mediated by different isoforms (MOR1 vs. MOR1D) which would have major therapeutic implications (Liu et al. 2011). Conversely, κ-opioid antagonists have been found to enhance itch (Kamei and Nagase 2001). In line with these results, the κ-opioid agonist nalbuphine has been shown to reduce μ-opioid-induced pruritus (Kjellberg and Tramer 2001) and has already been tested successfully in chronic itch patients using nalfurafine, a newly developed κ-opioid agonist (Kumagai et al. 2010).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


