Chapter 30 Antibacterial and antiviral drugs
Antibacterial and antiviral compounds constitute two of the groups of antimicrobial substances; other representatives are antiprotozoal drugs (Chapter 28) and antifungal drugs. Antimicrobial agents can also be categorized according to whether they are antibiotics (derived from the growth of microorganisms), chemotherapeutic agents (synthetic compounds not found in nature) or derivatives from non-microbial natural sources (lichens, higher plants, animals).
ANTIBACTERIAL DRUGS
Sources
History
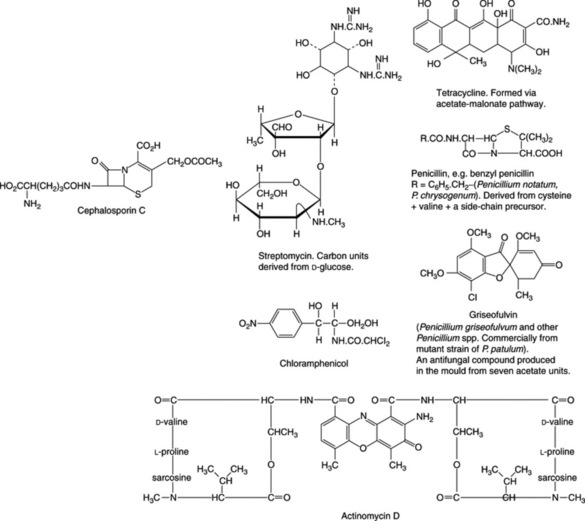
The advent of World War II launched a large-scale programme for the production and testing of the substance now known as penicillin, andthe resources of industry and academic institutions were devoted to the study of this substance and the search for other antibiotics. This led to the discovery of streptomycin, aureomycin, chloromycetin and many other antibiotics involving notably various species of Streptomyces. High-yielding strains of Penicillium chrysogenum which produce little pigment have now replaced P. notatum for penicillin manufacture. Preferential synthesis of benzylpenicillin is achieved by the addition of phenylacetic acid to the fermentation medium.
Clinical use
Of the antibiotics in clinical use, most are of bacterial or fungal origin (Table 30.1). Among the bacteria, the genus Streptomycesis particularly noteworthy, as it produces antibiotics such as streptomycin, chloramphenicol, chlortetracycline, tetracycline, erythromycin and neomycin. The penicillins, griseofulvin (an antifungal agent) and cephalosporins are of fungal origin.
Table 30.1 Some clinically important antibiotics
| Types and examples | Sources | Notes |
|---|---|---|
| Penicillins | Various Penicillium spp. | Based on β-lactam structure with various side-chains mainly at position 6 |
| Benzylpenicillin (Penicillin G) | Suitable strains of P. notatum | Acts by interfering with the synthesis of bacterial cell membranes. Inactivated by bacterially produced penicillinases |
| Phenoxymethyl penicillin (Penicillin V) | As above, with phenoxyacetic acid added to the culture medium | Unlike benzyl penicillin it is resistant to acid gastric juice |
| Semisynthetic penicillins | By enzymatic removal of side-chain of penicillin and re-esterification with other acids | Have modified properties of penicillin G such as acid resistance, penicillinase resistance (flucloxacillin), broad-spectrum activity (ampicillin) and antipseudomonal activity (ureidopenicillins) |
| Cephalosporins | Core structure similar to that of the penicillins and based on 7-aminocephalosporic acid | |
| Cephalosporin C | Cephalosporium acremonium | Has only moderate antibacterial activity |
| Modified cephalosporins | Side-chain substitution—see text | They have a higher degree of resistance to staphylococcal penicillinase compared with the penicillins. Wide spectrum of activity against Gram-negative bacteria |
| Tetracyclines | ||
| Tetracycline, chlortetracycline, oxytetracycline and others | Streptomyces spp., S. aureofaciens, S. rimosus | Broad-spectrum antibiotics to which bacterial resistance has greatly increased. Bacteriostatic rather than bactericidal. Most commonly prescribed for chronic bronchitis |
| Chloramphenicol | S. venezuelae: now by synthesis | Because of its toxicity chloramphenicol should be reserved for life-threatening diseases such as typhoid fever, meningitis, infections of Haemophilus influenzae and other conditions where no other antibiotic is effective. Widely used as eye drops |
| Aminoglycosides | Various soil organisms | All are bactericidal and active against many Gram-negative and some Gram-positive organisms |
| Streptomycin | Strains of Streptomyces griseus and other spp. | Discovered shortly after penicillin, streptomycin was used for the treatment of tuberculosis. Resistance was rapidly developed by the tubercle bacilli and it is now little used |
| Gentamicin | Micromonospora purpurea | The most widely used of the aminoglycosides; BP drug is a mixture of various gentamicin sulphates. Used in a variety of preparations |
| Neomycin | Selected strains of Streptomyces fradiae | Not used systemically. Administered prior to colonic surgery to suppress bowel flora. Topical applications. Eye preparations |
| Macrolides | ||
| Erythromycin | Certain strains of Streptomyces erythreus which produce principally erythromycin A | An alternative therapy for penicillin-hypersensitive patients |
| Nystatin | Streptomyces spp. | A polyene macrolide used for local treatment of the fungus Candida albicans |
| Peptides | Principally Bacillus spp. | Composed of a polypeptide chain linked to another group such as a long-chain fatty acid |
| Bacitracin | B. subtilis, B. licheniformis | In combination with other antibiotics it is used principally to treat skin infections |
| Polymyxin B | B. polymyxa | Effective against Gram-negative organisms particularly Pseudomonas aeruginosa. Included in bladder instillations, eye and ear drops and as other topical preparations |
| Colistan (Polymyxin E) | B. colistinus | Uses include bowel sterilization regimens |
| Cytotoxic antibiotics | ||
| Actinomycin D, daunorubicin and others | Various Streptomyces spp. | Anticancer therapy |
The cephalosporins (cefalosporins) are broad-spectrum antibiotics related both structurally and clinically to the penicillins. Being stable in acid solutions, some can be administered orally. Cephalosporin C arises by fermentation utilizing Cephalosporium acrimonium. As shown in Fig. 30.1, cephalosporin possesses two side-chains and substitution of these with a variety of groups has given rise to a considerable number of clinically effective drugs; some twelve, with theirpreparations, are listed in the current British National Formulary. They are usually graded, somewhat arbitrarily into first-, second- and third-generation cephalosporins, which roughly conform to the dates they were introduced and the particular type of derivative. Drugs from all generations are currently in use.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree