318. The answer is e. (Brunton, p 999; Katzung, p 656.) It has been said that the initial phase of uricosuric therapy is the most worrisome period for a hyperuricemic patient. Probenecid is a uricosuric drug (one that increases renal elimination of uric acid), but that effect is highly dose-dependent. Desired uricosuric actions depend on having therapeutic probenecid blood levels that are sufficient to inhibit active tubular reabsorption of urate. At subtherapeutic blood levels, the main effect is inhibition of tubular secretion of urate, which reduces net urate excretion and raises plasma urate levels (sometimes to the point of causing clinical gout). It is only once probenecid levels are therapeutic that the desired effects to inhibit tubular urate reabsorption (ie, increase excretion) predominate. Thus, and intuitively, once a patient starts probenecid therapy drug levels must rise and pass through that stage in which urate excretion will actually go down, plasma urate levels up.
Some texts suggest using a short course of colchicine or another (non-aspirin) NSAID that is indicated for gout when probenecid therapy is started. That is for prophylaxis of acute gout that might occur. Although this may be acceptable, other rules are perhaps more important: (1) do not administer a uricosuric during a gout attack; (2) if the patient has had a gout attack recently, suppress the inflammation for 2 to 3 months with a suitable anti-inflammatory; and (3) do not use uricosurics for patients with “severe hyperuricemia” and/or poor renal function. Doing otherwise is associated with a great risk of potentially severe renal tubular damage as the uricosuric shifts large amounts of uric acid from the blood (with its large volume, that keeps urate relatively “dilute”) into a small volume of acidic urine, which concentrates urate and lowers its solubility via pH-dependent mechanisms.
(The patient who skips doses of probenecid also becomes very vulnerable to the “paradoxical” extra risk, because doing this may allow drug levels to fall into that subtherapeutic range in which more urate is retained than eliminated.)
319. The answer is d. (Brunton, pp 975-976.) Before the warning was issued, over 500 children with any viral illness and who received aspirin (even in usual recommended doses at the time) developed Reye syndrome. It is characterized by encephalopathy and fatty liver degeneration. The mortality rate ranged from about 20% to 30%, and many more patients developed permanent and serious CNS and/or liver dysfunction. Nowadays there are fewer than five cases of Reye syndrome per year as a result of the aspirin/viral illness “interaction.” (Note: the age range for which aspirin should not be administered in the presence of viral illness is debatable; some suggest the risk of Reye syndrome lessens dramatically by the age of 12 years, but many experts suggest that the risk may persist up to 18-20 years of age.)
Asthma (a) is the most common cause of hospitalizations in children, and many patients have their asthma provoked or worsened by aspirin or other NSAIDs. However, the potential asthma—NSAID problems are unrelated to the presence or absence of viral illnesses. There is no evidence of a linkage between aspirin and cardiomyopathies (b). Long-term high dose administration of NSAIDs (other than aspirin) may cause renal dysfunction or failure (c). Here, too, there is no linkage between viral illness, and the kidney problems are not at all focused on the pediatric population. Aspirin obviously has antiplatelet-aggregatory effects, but whether the patient is a child or older, thrombocytopenia (e) is not a concern.
320. The answer is e. (Brunton, p 960; Katzung, pp 643-648.) Methotrexate, gold salts, and penicillamine are members of a diverse group of drugs called DMARDs (disease-modifying antirheumatic drugs) or SAARDs (slow-acting antirheumatic drugs). The former term derives from the ability of these drugs to slow, stop, or in some cases reverse joint damage associated with rheumatoid arthritis (RA). They do more than merely mask or relieve RA symptoms, which is mainly what the traditional NSAIDs do. The second acronym derives from the fact that it may take a month (or a couple more) for meaningful symptom relief to develop; they are not at all quick-acting drugs. Their actions probably are due to suppression of immune responses that often contribute to the etiology of RA.
Their toxicities can be serious, which is one reason why, until not long ago, these agents were considered third-line or even last resort treatments for refractory rheumatoid disease. (Methotrexate is now being used much earlier, and safely, for RA, now that we know better how to use it and monitor for serious toxicities.) Most of these drugs can cause serious blood dyscrasias; in addition, penicillamine can cause renal and pulmonary toxicity; hydroxychloroquine is associated with vision impairments/retinopathy.
321. The answer is a. (Brunton, pp 941-942, 964-965; Katzung, p 640.) Celecoxib, by virtue of selective COX-2 inhibition, does not interfere as much with synthesis of PGE2, which normally suppresses a component of gastric acid secretion and stimulates gastric mucus production. Overall, then, the risks of gastric and duodenal ulcers are reduced. The selectivity also means that COX-2 inhibitors do not interfere with the production of other eicosanoids, such as TXA2. That is both good and bad, clinically. On the good side, this means that COX-2 inhibitors don’t cause antiplatelet effects and increase the risk of excessive or spontaneous bleeding. On the other hand, this lack of effect renders them unsuitable for causing desired antiplatelet-aggregatory effects, as might be wanted when we administer aspirin. This may explain the finding that some COX-2 inhibitors have been associated with (cause?) a higher risk of sudden cardiac death (d) in vulnerable patients, and why some have been pulled off the market (and are subjects of considerable litigation).
Selective COX-2 inhibitors, such as the nonselective alternatives, aren’t cures for arthritis (b); they alleviate signs and symptoms but seem to have no demonstrable impact on the underlying pathophysiology. They have no effect on uric acid metabolism (c) or excretion. Their onsets of action are, overall, no faster (e) than those of a typical NSAID.
322. The answer is d. (Brunton, pp 937-939; Katzung, pp 339-340, 347-348, 639f.) Anti-inflammatory doses of glucocorticosteroids inhibit phospholipase A2 activity. In doing so, they inhibit arachidonic acid synthesis and, therefore, synthesis of all subsequent products of the cyclooxygenase and lipoxygenase pathways, both of which originate with arachidonic acid. This action of glucocorticoids is indirect because their initial or direct effect is induced synthesis of annexins (previously called lipocortins), which are the moieties that directly inhibit PLA2 activity. Glucocorticoids have no intrinsic or direct inhibitory effects on later steps in AA metabolism, that is, no direct effects on cyclooxygenase or lipoxygenase activity.
Cyclooxygenases (a) are inhibited by traditional NSAIDs such as aspirin (nonselective COX-1 and -2 inhibitors), or the “coxibs” (celecoxib), which relatively selectively inhibit COX-2. We have no clinically useful inhibitors of histamine synthesis (which involves histidine decarboxylase activity; b). As noted elsewhere, zileuton inhibits 5′-lipoxygenase (c) and the subsequent formation of leukotrienes. Allopurinol inhibits the synthesis of xanthine and uric acid by inhibiting xanthine oxidase (e). The same applies to the new allopurinol alternative, febuxostat.
323. The answer is a. (Brunton, pp 721-725, 932-933; Katzung, pp 184-185.) Bradykinin is metabolized to biologically inactive peptides by an enzyme that has at least three names: angiotensin-converting enzyme (ACE; recall that the prototype ACE inhibitor is captopril), bradykininase, and kininase II. The name that is used depends on the substrate. When the substrate is angiotensin I, the enzyme is called ACE. When the substrate is bradykinin we call it either bradykininase or kininase II.
Bradykinin, whether injected experimentally or derived from endogenous sources (kininogens cleaved by specific proteases called kallikreins), exerts significant vasodilator effects that can lower systolic and diastolic blood pressures. Although this may not be an important pressure-regulating mechanism in normotensive individuals, it probably is in many (most?) patients with essential hypertension. Clearly, bradykinin does not increase blood pressure (c) or constrict the renal vasculature (e).
Recall that ACE inhibitors lower blood pressure in many hypertensive patients. One mechanism involves “preserving” bradykinin by inhibiting its enzymatic inactivation. Bradykinin also causes prerenal arteriolar vasodilation and increases GFR, leading to diuretic effects.
The peptide’s vascular effects are mediated by endothelial cell-derived nitric oxide, and they are enhanced (not counteracted, b) by other drugs that cause vasodilation by a nitric oxide-related mechanism.
Bradykinin receptor blockers prevent the peptide’s vasodilator effects. However, no currently approved drugs, including the so-called second-generation antihistamines such as fexofenadine (d) exert that effect.
324. The answer is c. (Brunton, p 966t; Katzung, p 641.) The ductus arteriosus in neonates may remain patent largely because of the vasodilator effects of endogenous PGE1, formed via the cyclooxygenase pathway. When the goal is to close a patent ductus after birth we generally use a prostaglandin synthesis (COX-1/-2) inhibitor (indomethacin, historically; or ibuprofen more often nowadays). (Conversely, there are times when surgical procedures are required on a congenitally anomalous heart in newborns, and we want to keep the ductus open until surgery. In that case, alprostadil [PGE1, answer e] may be administered.)
Misoprostol (d) is a prostaglandin analog. Giving it to a newborn with a patent ductus is likely to keep the lesion open, not close it. None of the H2 blockers (eg, cimetidine, a) nor H1 blockers (diphenhydramine, b, and many others) have any important effects on prostaglandin synthesis, nor are they used either to close a patent ductus arteriosus or to keep it open for subsequent surgery.
325. The answer is d. (Brunton, pp 399, 405, 410-413, 1537; Katzung, pp 285f, 292, 533-537, 1161t.) This patient has what is almost certainly the serotonin syndrome. The triptan “adds” serotonin to serotoninergic synapse, and serotonin’s neuronal reuptake will be blocked by fluoxetine (or sertraline, others), which is classified as a selective serotonin reuptake inhibitor (SSRI) antidepressant. When sumatriptan (or other triptans used for migraine) is added, rapid accumulation of serotonin and/or the triptan in the brain synapses can occur.
The other drugs listed (acetaminophen, a; codeine, b; diazepam, c; and phenytoin, e) are not likely to interact with serotoninergic drugs.
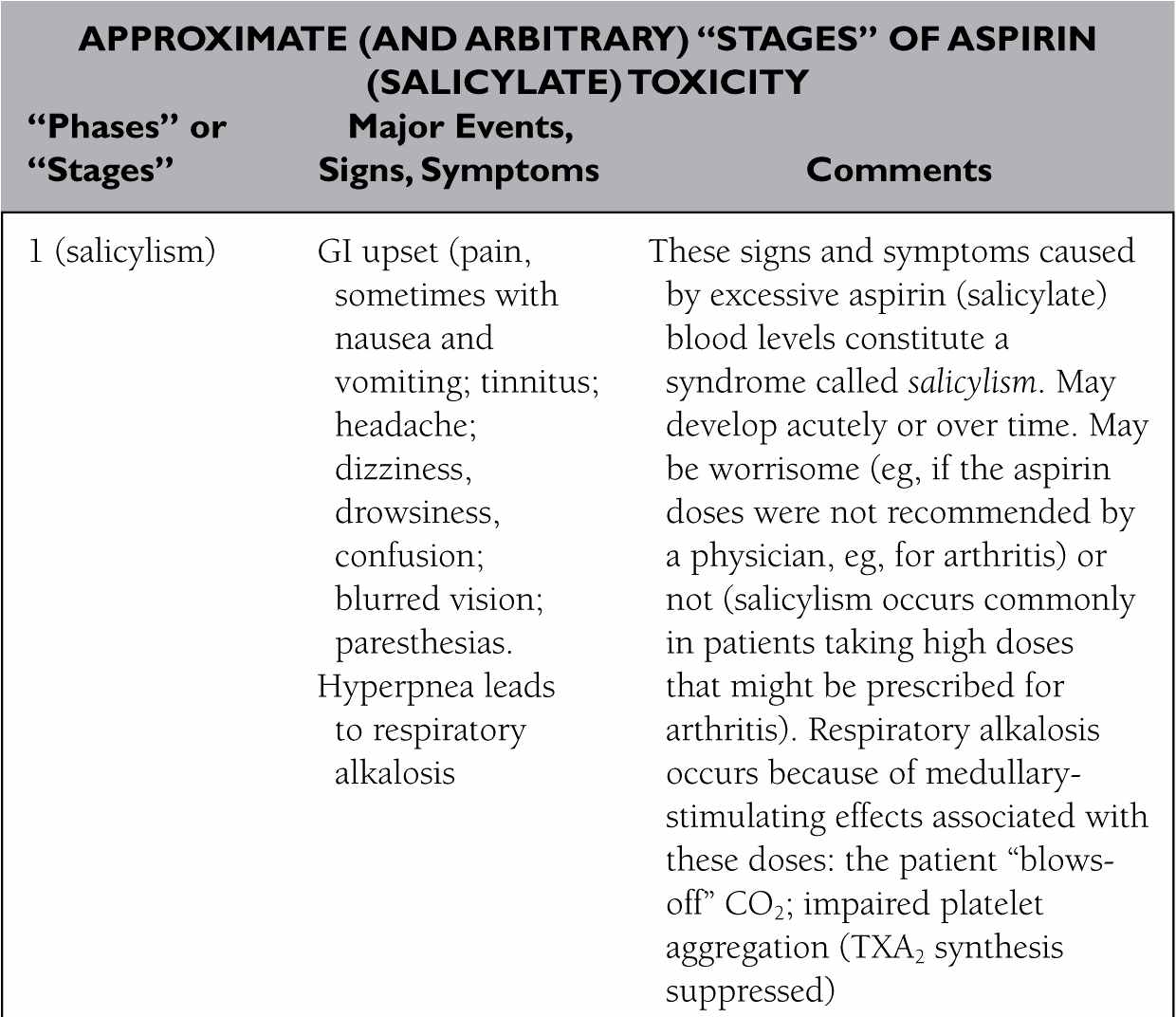
326. The answer is e. (Brunton, pp 75, 83t, 977-982; Katzung, pp 62, 1034-1035.) Tinnitus, along with a feeling of dizziness or lightheadedness, GI upset (including nausea and some pain or other discomfort, and diarrhea more so than constipation), and such visual changes as blurred or diplopia (but not myopia, d), all are part of a low-grade aspirin “toxicity” syndrome called salicylism. It is not necessarily worrisome (provided the physician intentionally prescribed the drug at dosages likely to produce the syndrome), dangerous, or indicative of imminent and severe toxicity. Indeed, some patients experience one or more signs or symptoms of salicylism in response to high (antiarthritic) doses of aspirin.
Constipation (a) or hypertension (c) are not causally associated with salicylate administration, regardless of the dose used. Cough (b) may occur, but that is only likely to occur in patients who have “aspirin-sensitive” asthma, and for those patients bronchoconstriction is more likely than cough to be the response.
327. The answer is e. (Brunton, p 999; Katzung, pp 656, 1160t.) Probenecid (and the related drug, sulfinpyrazone) are classified as uricosurics: at sufficiently high (therapeutic) doses, they enhance the renal elimination of uric acid by inhibiting tubular reabsorption of filtered urate. Aspirin significantly impairs the uricosurics’ actions.
Important note: Aspirin (given alone) has blood level-dependent effects on urate elimination by the kidneys. At “low doses” perhaps up to about 1 g/day, it selectively inhibits tubular secretion of urate and so can raise plasma urate levels. At doses much higher than that (including doses sometimes prescribed for arthritis other than gout), the predominant effect (and the net, or overall, effect) is uricosuria due to blockade of tubular reabsorption of urate (this effect is greater than the drug’s inhibitory effect on tubular secretion of urate). Nonetheless, aspirin is not used as a uricosuric drug because the doses/blood levels needed to increase urate elimination are sufficiently high that they cause significant side effects (eg, salicylism; see Question 326) that don’t arise with the traditional uricosurics such as probenecid.
Acetaminophen (a) has no appreciable or clinically useful effects on uric acid elimination or synthesis. Allopurinol (b, and the new related drug febuxostat) inhibits uric acid synthesis, but its xanthine oxidase-inhibitory effects are not altered by aspirin. However, since allopurinol is used to lower plasma urate levels it is largely irrational to use aspirin at the same time. The same applies to colchicine (c) and indomethacin (d), both of which are used for in acute gout and neither of which has its main biochemical effects (suppression of inflammation) impaired by aspirin.
328. The answer is c. (Brunton, pp 952, 1837, 1849; Katzung, pp 315, 327, 1090.) In addition to misoprostol’s use as a cytoprotective/mucotropic drug for some patients taking NSAIDs, misoprostol is also used as an adjunct to mifepristone to induce therapeutic abortion. This capitalizes on the prostaglandin analog’s strong uterine-stimulating effects (thus, answer e is incorrect). The drug is sometimes used to maintain patency of an open ductus arteriosus, not to close it (a). It is not a contraceptive in the typical sense, such as we would associate with estrogen-progestin oral contraceptives. Given the drug’s abortifacient effects, it is contraindicated for women who are pregnant, or wish to become pregnant (b). Misoprostol has bronchodilator activity, but it is relatively weak and the drug is not suitable as a substitute for corticosteroids (d) or other typical asthma medications.
329. The answer is e. (Brunton, pp 75-76, 86t, 983; Katzung, pp 1034-1035.) Adjunctive use of sodium bicarbonate (IV) can be an important adjunct to managing severe salicylate poisoning for two main reasons: (1) it helps raise blood pH, which as stated earlier is profoundly reduced from metabolic plus respiratory acidosis; (2) it alkalinizes the urine, which (via a pH-dependent mechanism, a la Henderson-Hasselbach) converts more aspirin molecules into the ionized form in the tubules, thereby reducing tubular reabsorption of a substance we want to eliminate from the body as quickly as possible.
Acetaminophen (a), even though it is usually an effective antipyretic, is not good for managing fever of severe aspirin poisoning. It would add yet another drug that might complicate the clinical picture, and ordinary (and ordinarily safe) doses aren’t likely to do much to lower temperature quickly or sufficiently. (Thus, we use physical means to lower body temperature.) N-acetylcysteine (c), the antidote for acetaminophen poisoning, does nothing for salicylate poisoning. Amphetamines (b) might seem rational for managing ventilatory depression that characterizes late stages of severe aspirin poisoning. The more likely outcome of giving an amphetamine is simply to hasten the onset of seizures. Phenobarbital (d), or other CNS depressants, would aggravate an already bad state of CNS/ventilatory depression. (However, if seizures develop they must be managed—eg, with IV lorazepam and phenytoin, even though they cause CNS depression. Without them, the patient may quickly die from status epilepticus.)
Just as I provided a table (answer to Question 317) to summarize the general sequence of events in acetaminophen toxicity, I’ll provide one here for aspirin overdoses. Again, the “stages” I present are arbitrary, but they should be helpful—knowing what is likely to come next as toxicity progresses helps you prepare for what you’ll probably need to do next.
Note that the average lethal dose for an otherwise healthy child is between around 5 g and 8 g, taken all at once. For adults, it is between around 10 g and 30 g. “Usual strength” aspirin tablets—those used for aches, pains, mild inflammation, and fever—contain 325 mg of the drug. (“Heart dose” aspirin tablets contain 81 mg.) Higher doses may be prescribed for arthritis—several grams a day—and although these usually are not sufficient to cause death they are likely to cause side effects that often are disturbing enough that the physician will prescribe another antiarthritic drug instead of plain, cheap, aspirin.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree