Anatomy of the Portal System and Experience with Portacaval Shunt
Marshall J. Orloff
Introduction
The excellent chapter on Anatomy of the Portal System by Drs. Richard Bell and Alan Koffron in the 5th edition of Mastery of Surgery bears repeating in substantial part. Therefore, much of it is presented in the 6th edition. It is supplemented by a detailed description of the side-to-side portacaval shunt (PCS) and a section titled “Author’s Experience with Portacaval Shunt,” based on the experience of the author with over 3,000 PCSs over a period of 52 years.
The portal vein arises from the postduodenal plexus of the embryonic vitelline veins. Rarely, the preduodenal plexus persists, giving rise to a portal vein that lies anterior to the duodenum. This anomaly, which is potentially lethal if it is not recognized and the portal vein is transected during surgery in this area, is associated with annular pancreas, malrotation, and biliary tract anomalies.
In the adult, the portal vein and its tributaries have no valves – those that existed during fetal circulation having been resorbed. Thus, the portal system can be decompressed at any point by creating a connection to the systemic venous system. The vein delivers blood from the spleen, the pancreas, and the digestive tube to the liver. It provides ∼75% of hepatic blood flow and 50% of the oxygen delivery to this organ.
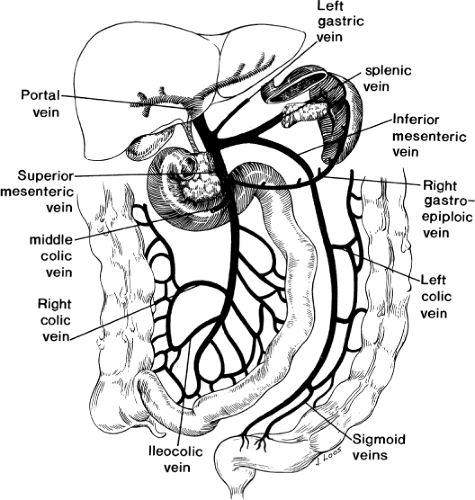
The portal vein can be thought of as the trunk of a tree, with the visceral veins (superior mesenteric, inferior mesenteric, and splenic) forming the roots, and the intrahepatic portions of the right and left portal veins forming the branches (Fig. 1). The vascular delta formed by the mixing of portal venous and hepatic arterial blood in the sinusoids of the liver then drains into the inferior vena cava (IVC) through the three hepatic veins.
The portal vein trunk is 4.8 to 8.8 cm long, with an average length of 6.4 cm, and 0.6 to 1.2 cm wide, with an average width of 0.9 cm. It is formed behind the neck of the pancreas, at the level of the second lumbar vertebra, by the confluence of the superior mesenteric vein (SMV) and the splenic vein (Fig. 2). In approximately one-third of the population, the inferior mesenteric vein joins the portal vein directly at this point, forming a trifurcation; in the remaining cases, it drains into the splenic vein (38%) or the SMV (29%). The portal vein then passes to the right and cephalad behind the first portion of the duodenum and into the hepatoduodenal ligament, which forms the ventral boundary of the foramen of Winslow. Within the hepatoduodenal ligament, the portal vein lies posterior to the hepatic artery and common bile duct, usually slightly to the left of the duct. It bifurcates in the porta hepatis at the right aspect of the hilar plate – a thickening of the liver’s fibrous capsule at the hilum. The right branch, which supplies the right hepatic lobe, is shorter (0.5 to 1.0 cm long), wider, and more variable than its sinistral counterpart. It often divides into anterior and posterior branches at its point of entry into the liver parenchyma. The more constant and longer left portal vein, with a 4-cm average length, supplies the left hepatic lobe and runs left in the hilar plate as the pars transversa until it enters the fissure for the ligamentum venosum. At this point, the vein receives the attachment of the ligamentum venosum and then curves anteriorly, becoming the pars umbilicus, which ends by attaching to the ligamentum teres hepaticus in the umbilical fissure. The caudate lobe is supplied by two to three branches arising from the bifurcation of the portal vein or from its right or left branches.
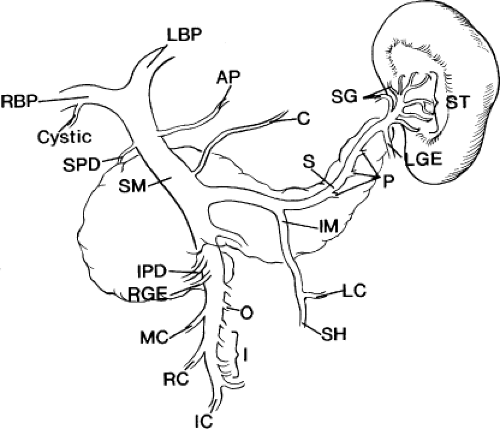
Of great importance to the surgeon are the sundry side branches that feed into the
portal vein as it forms behind the pancreas and runs cephalad in the hepatoduodenal ligament (Fig. 2). The portal vein usually receives the pancreaticoduodenal vein(s) and the pyloric vein, which is also known as the right gastric vein. More variable in their point of insertion is the coronary vein, which is also known as the left gastric vein, and the accessory pancreatic vein. The superior pancreaticoduodenal vein usually enters the right aspect of the portal vein at or just above the superior margin of the pancreas. The inferior pancreaticoduodenal vein usually enters the right aspect of the SMV just before it joins the splenic vein to form the portal vein proper. In ∼38% of cases, there is only a single pancreaticoduodenal vein, which joins the portal vein at the same juncture as the superior pancreaticoduodenal vein. The pyloric vein is present in 80% of the population and, in 75% of the population, terminates in the anterior aspect of the portal vein (inside the hepatoduodenal ligament), within 3.0 cm of the portal vein bifurcation. The coronary vein inserts at the superior aspect of the junction of the splenic and SMVs in 60% of the population, in the portal vein proper in 25% of the population, and in the splenic vein (just before its junction with the SMV) in 15% of the population. In a series of 92 dissections, 29 specimens had an accessory pancreatic vein terminating in the superior aspect of the midportal vein.
portal vein as it forms behind the pancreas and runs cephalad in the hepatoduodenal ligament (Fig. 2). The portal vein usually receives the pancreaticoduodenal vein(s) and the pyloric vein, which is also known as the right gastric vein. More variable in their point of insertion is the coronary vein, which is also known as the left gastric vein, and the accessory pancreatic vein. The superior pancreaticoduodenal vein usually enters the right aspect of the portal vein at or just above the superior margin of the pancreas. The inferior pancreaticoduodenal vein usually enters the right aspect of the SMV just before it joins the splenic vein to form the portal vein proper. In ∼38% of cases, there is only a single pancreaticoduodenal vein, which joins the portal vein at the same juncture as the superior pancreaticoduodenal vein. The pyloric vein is present in 80% of the population and, in 75% of the population, terminates in the anterior aspect of the portal vein (inside the hepatoduodenal ligament), within 3.0 cm of the portal vein bifurcation. The coronary vein inserts at the superior aspect of the junction of the splenic and SMVs in 60% of the population, in the portal vein proper in 25% of the population, and in the splenic vein (just before its junction with the SMV) in 15% of the population. In a series of 92 dissections, 29 specimens had an accessory pancreatic vein terminating in the superior aspect of the midportal vein.
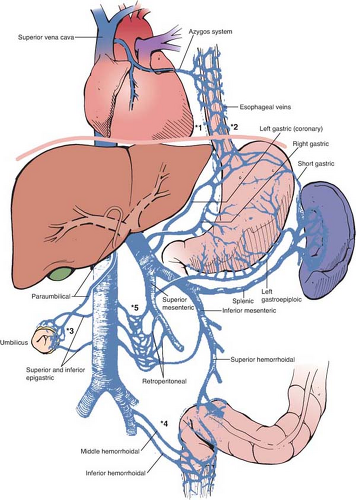
Another anatomic aspect of the portal system that is of importance to the surgeon are the points of communication between the portal and systemic (caval, azygous, and hemiazygous) venous systems (Fig. 3). Normally, these anastomotic channels are small, but the combination of portal hypertension and the lack of valves in the adult portal system lead to reversal of flow, with consequent dilatation and tortuosity. Clinically, the most important portosystemic anastomoses are the veins of the proximal stomach and distal esophagus, which receive flow from the coronary and short gastric veins and drain into the superior vena cava via the azygous system (labeled *1 and *2 in Fig. 3). Dilatation and tortuosity in this plexus of relatively unsupported veins results in varices that can rupture, causing exsanguinating hemorrhage. Other significant anastomotic areas shown in Fig. 3 include the following:
The submucosal venous plexus in the rectum between the superior hemorrhoidal veins (the portal system) and the middle and inferior hemorrhoidal veins (the caval system) (labeled *4). Large bleeding rectal varices, although uncommon, can arise here in patients with portal hypertension.
The paraumbilical veins, which connect the left portal vein via a recanalized umbilical vein to the epigastric venous network of the abdominal wall, which drains into the caval system (labeled *3). This plexus can become the variceal “caput Medusae” in patients with portal hypertension and produce the Cruveilhier–Baumgarten syndrome. Unintentional transection of these varices during surgery that involves the anterior abdominal wall can result in significant blood loss.
Retzius veins: a group of small but numerous retroperitoneal veins that connect abdominal viscera, both tubular and solid, with the caval system via intercostals, phrenic, lumbar, and renal veins (labeled *5). In the patient with portal hypertension, bleeding from these veins can make any upper abdominal operation dangerous depending on the amount and location of retroperitoneal dissection required.
Finally, significant venous collaterals can form in surgical adhesions, making reoperative surgery and stomal takedowns more dangerous in patients with portal hypertension.
Portosystemic Shunts
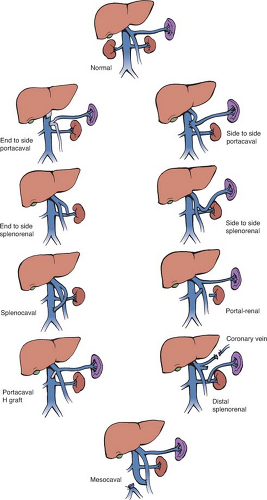
One of the common categories of surgical operations in which a detailed knowledge of portal anatomy is essential is that of portosystemic shunts. Because the portal venous system contains no valves, it is possible to decompress the system and relieve portal hypertension by connecting the portal vein or its tributaries to the systemic venous system at a number of sites, provided the systemic vein is of sufficient size to handle the large portal blood flow. Figure 4 shows various types of portosystemic shunts. Not shown is the transjugular intrahepatic portosystemic shunt (TIPS), which is a radiologic procedure that creates a side-to-side shunt within the liver. A detailed review of the various causes of portal hypertension and the advantages and disadvantages of the several kinds of shunts is beyond the scope of this chapter. Rather, the anatomic points of portal vein anatomy relevant to performance of a side-to-side portacaval shunt (SSPCS) are discussed since, in the large experience of the author with the treatment of portal hypertension, the direct SSPCS has been used in over 90% of the patients.
The indications for a SSPCS are (a) bleeding esophageal or gastric varices due to portal hypertension caused by cirrhosis of the liver
or, much less commonly, by other liver diseases such as schistosomiasis; (b) bleeding from portal hypertensive gastropathy unresponsive to pharmacologic therapy; (c) Budd–Chiari syndrome (BCS) with occluded hepatic veins and patent IVC; (d) intractable ascites unresponsive to nonsurgical therapy; and (e) failed TIPS.
or, much less commonly, by other liver diseases such as schistosomiasis; (b) bleeding from portal hypertensive gastropathy unresponsive to pharmacologic therapy; (c) Budd–Chiari syndrome (BCS) with occluded hepatic veins and patent IVC; (d) intractable ascites unresponsive to nonsurgical therapy; and (e) failed TIPS.
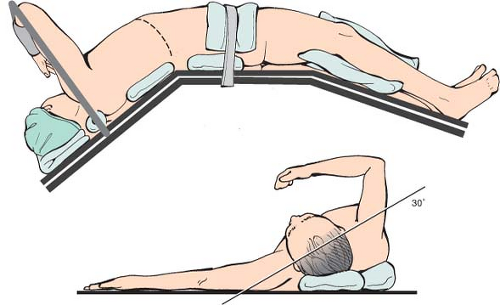
The position of the patient on the operating table is crucial and can make the difference between an easy and difficult operation (Fig. 5). Specifically, the patient is placed on the operating table with the right side elevated at an angle of 30 degrees to the table, and the table is adjusted so as to widen the space between the right costal margin and right iliac crest, and make it possible to perform the operation easily through a long right subcostal incision.
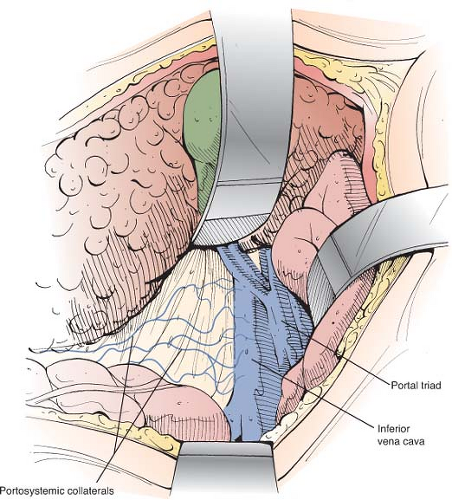
Once inside the peritoneal cavity, retractors are placed and the posterior peritoneum overlying the IVC is incised with electrocautery by an extended Kocher maneuver (Fig. 6). The anterior surface of the IVC is cleared of fibroareolar tissue, and the IVC is isolated around its entire circumference by blunt and sharp dissection from the entrance of the right and left renal veins, below, to the point where it disappears behind the liver, above. The IVC is encircled with an umbilical tape (Fig. 7). To accomplish the isolation, several tributaries must be ligated in continuity with fine silk ligatures and then divided. These tributaries often include the right adrenal vein, one or two pairs of lumbar veins that enter the posterior surface, and the caudal pair of small hepatic veins from the caudate lobe of the liver that enter the anterior surface of the IVC directly from the liver.
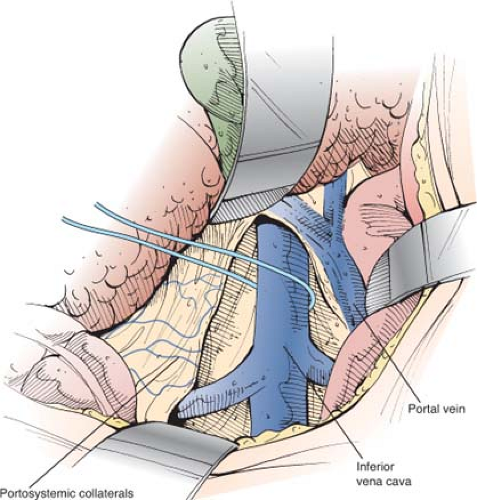
When the IVC has been mobilized completely, it can be lifted up toward the portal vein (Fig. 8). Failure to isolate the IVC circumferentially is one major reason for the erroneous claim that the SSPCS often cannot be performed because the portal vein and IVC are too widely separated.
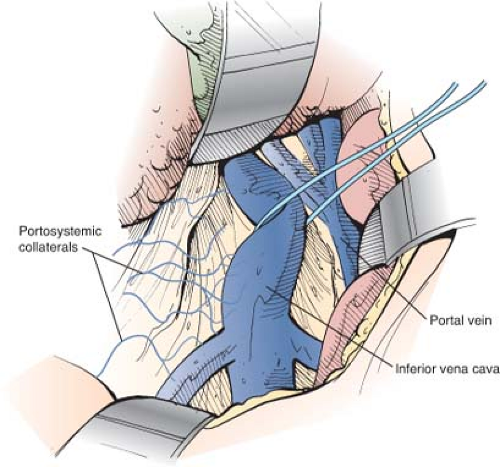
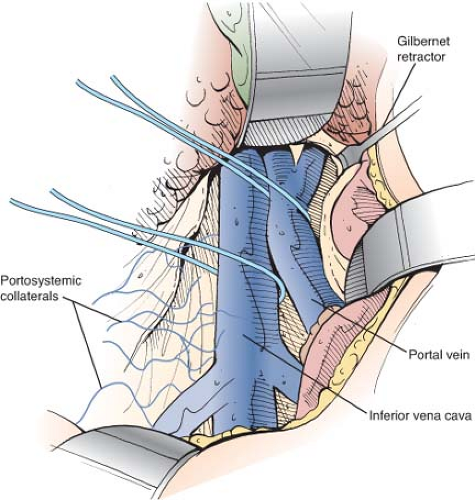
The superior retractor is repositioned medially so that it retracts the liver at the point of entrance of the portal triad. The portal vein is located in the posterolateral aspect of the portal triad and is approached from behind. The fibrofatty tissue on the posterolateral aspect of the portal triad, which contains nerves, lymphatics, and lymph nodes, is divided by blunt and sharp dissection. This technique is a safe maneuver because there are no portal venous tributaries on this aspect of the portal triad. As soon as the surface of the portal vein is exposed, a vein retractor or Gilbernet retractor is inserted to retract the common bile duct medially. The portal vein is mobilized circumferentially at its midportion and is encircled with an umbilical tape (Fig. 9). It is then isolated up to its bifurcation in the liver hilum. Several tributaries on the medial aspect are ligated in continuity with fine silk and divided.
Using the umbilical tape to pull the portal vein out of its bed, the portal vein is
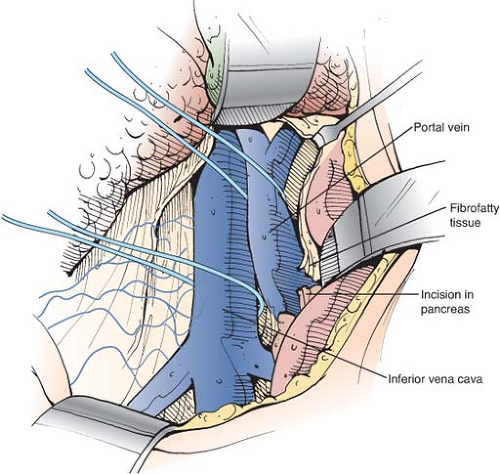
cleared to the point where it disappears behind the pancreas. The tough fibrofatty tissue that binds the portal vein to the pancreas must be divided (Fig. 10). Several tributaries that enter the medial aspect of the portal vein and one tributary that enters the posterolateral aspect are divided. It is usually not necessary to divide the splenic vein. Wide mobilization of the portal vein is essential for performance of a side-to-side portacaval anastomosis. Failure to mobilize the portal vein behind the pancreas is a second major reason for difficulty in accomplishing the side-to-side shunt. In some patients, it is necessary to divide a bit of the head of the pancreas between right-angled clamps to obtain adequate mobilization of the portal vein. Bleeding from the edges of the divided pancreas is controlled with suture ligatures. Division of a small amount of the pancreas is a very helpful maneuver and we have never observed postoperative complications, such as pancreatitis, from its performance. Before incising the pancreas, the surgeon should insert his index finger into the tunnel between the portal vein and the pancreas to determine by palpation if there is a replaced common hepatic or right hepatic artery arising from the superior mesenteric artery and crossing the portal vein. Since the portal venous blood flow to the liver is diverted through the PCS, ligation of the hepatic arterial blood supply may be lethal.
cleared to the point where it disappears behind the pancreas. The tough fibrofatty tissue that binds the portal vein to the pancreas must be divided (Fig. 10). Several tributaries that enter the medial aspect of the portal vein and one tributary that enters the posterolateral aspect are divided. It is usually not necessary to divide the splenic vein. Wide mobilization of the portal vein is essential for performance of a side-to-side portacaval anastomosis. Failure to mobilize the portal vein behind the pancreas is a second major reason for difficulty in accomplishing the side-to-side shunt. In some patients, it is necessary to divide a bit of the head of the pancreas between right-angled clamps to obtain adequate mobilization of the portal vein. Bleeding from the edges of the divided pancreas is controlled with suture ligatures. Division of a small amount of the pancreas is a very helpful maneuver and we have never observed postoperative complications, such as pancreatitis, from its performance. Before incising the pancreas, the surgeon should insert his index finger into the tunnel between the portal vein and the pancreas to determine by palpation if there is a replaced common hepatic or right hepatic artery arising from the superior mesenteric artery and crossing the portal vein. Since the portal venous blood flow to the liver is diverted through the PCS, ligation of the hepatic arterial blood supply may be lethal.
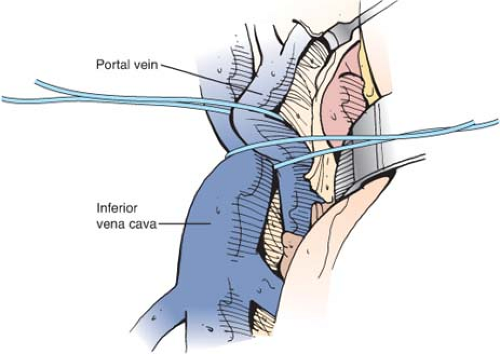
To determine the adequacy of mobilization of portal vein and IVC, the two vessels are brought together by traction on the umbilical tapes that surround them (Fig. 11). It is essential to determine that the two vessels can be brought together without excessive tension. If this cannot be done, it is almost always because the vessels have not been adequately mobilized, and further dissection of the vessels should be undertaken. Resection of part of an enlarged caudate lobe of the cirrhotic liver, recommended by some surgeons to facilitate bringing the vessels together, is associated with some difficulties and, in our opinion, is neither necessary nor advisable.
Pressures in the IVC and portal vein are measured with a saline (spinal) manometer by direct needle puncture before performance of the portacaval anastomosis. A portal vein-IVC pressure gradient of 150-mm saline or higher represents clinically significant portal hypertension. Most patients with bleeding esophageal varices (BEV) have a portal vein-IVC gradient of 200-mm saline or higher.
The steps in performing the side-to-side portacaval anastomosis are as follows:
A Satinsky clamp is placed obliquely across a 5-cm segment of the anteromedial wall of the IVC in a direction parallel to the course of the overlying portal vein and the IVC is elevated toward the portal vein. A 5-cm segment of the portal vein is isolated between two angled vascular clamps and the portal vein is depressed toward the IVC, bringing the two vessels into apposition (Fig. 12).
A 2.0- to 2.5-cm long strip of the IVC and a 2.0- to 2.5-cm long strip of the portal vein are excised with scissors (Fig. 13). It is important to excise a longitudinal segment of the wall of each vessel rather than simply making an incision in each vessel. A retraction suture of 5/0 silk is placed in the lateral wall of the IVC opening and is weighted by attachment to a hemostat to keep the IVC orifice open. The clamps on the portal vein are momentarily released to flush out any clots and then the openings in both vessels are irrigated with saline.
The anastomosis is started with a posterior continuous over-and-over suture of 5/0 vascular suture material. The posterior continuous suture is tied at each end of the anastomosis.
The anterior row of sutures consists of an everting continuous horizontal mattress stitch of 5/0 vascular suture material started at each end of the anastomosis (Fig. 14). The suture started at the inferior end of the anastomosis is discontinued after three or four throws and is deliberately left loose so that the interior surface of the vessels can be visualized as the anastomosis is completed. In this way inadvertent inclusion of the posterior wall in the anterior row of sutures is avoided. The suture started at the superior end of the anastomosis is inserted with continuous tension until it meets the inferior suture, at which point the inferior suture is drawn tight and the two sutures are tied to each other. Before drawing the inferior suture tight, the clamps on the portal vein are momentarily released to flush out any clots, and the anastomosis is thoroughly irrigated with saline.
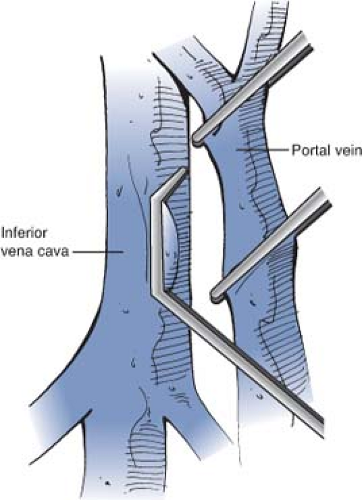
Fig. 12. Clamping the IVC and portal vein in preparation for a side-to-side portacaval anastomosis. A Satinsky clamp is placed obliquely across a 5-cm segment of the anteromedial wall of the IVC in a direction parallel to the course of the overlying portal vein and the IVC is elevated toward the portal vein. A 5-cm segment of the portal vein is isolated between two angled vascular clamps and the portal vein is depressed toward the IVC, bringing the two vessels into a position.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access