Anatomy and Physiologic Morphology
SYED A. HODA
EMBRYOLOGY AND DEVELOPMENT OF THE IMMATURE BREAST
Embryology
The breasts develop from the mammary ridges or milk lines, which are thickenings of the epidermis that first appear on the ventral surface of the 5-week fetus. These ridges extend from the axilla to the upper medial region of the thigh. In humans, most of the ridge does not develop further and disappears during fetal development. Persistence of segments of the milk line is the embryologic anlage for ectopic mammary glandular tissue, which occurs most often at the extreme ends of the mammary ridge in the axilla or vulva. Molecular mechanisms guiding embryonic mammary gland development and the potential role of stem cells in normal mammary development and maintenance have been reviewed by Cowin and Wysolmerski1 and van Keymeuelen et al.,2 respectively.
Mesenchymal condensation occurs around an epithelial stalk, the breast bud, at the site of mammary development on the chest wall in the 15th week of gestation. Growth of cords of epithelium into the mesenchyme produces a group of solid epithelial columns, each of which gives rise to a lobe in the mammary gland. The papillary layer of the fetal dermis continues to encase these growing epithelial cords, and it ultimately evolves into the vascularized fibrous tissue surrounding individual ducts and their branches of ducts that form lobules. In the fetal breast, epithelial cells that form the breast bud express transforming growth factor α (TGF-α), a mitogen and differentiation factor that may mediate the growth-promoting effect of estrogen on the developing breast.3 Stromal tissue surrounding the breast bud is rich in TGF-β1, a protein involved in modulating cell-matrix interactions. The basement membrane protein, collagen type IV, is distributed around the basal layer of cells in the breast bud. Early in fetal development, proliferative activity measured by Ki67 immunoreactivity is maximal in the region of the neck of the breast bud, involving epithelial and stromal cells. The development of the fetal breast is characterized by the differential expression of keratins 14, 18, and 19 and of actin in the breast ducts and lobular buds.4
Myoepithelial cells appear to arise from basal cells between weeks 23 and 28 of gestation.5 They play an important role in the branching morphogenesis of the mammary gland through the synthesis of basement membrane constituents such as laminin, type IV collagen, and fibronectin, as well as metalloproteinases and growth factors.6
Less cellular, more collagenized stroma that originates in the reticular dermis extends into the breast to encompass lobes and subdivisions of lobes, forming the suspensory ligaments of Cooper that attach the breast parenchyma to the skin.5 Coincidentally, differentiation of the mesenchyme into fat within the collagenous stroma occurs between weeks 20 and 32. In the last 2 months of gestation, canalization of the epithelial cords occurs, followed by the development of branching lobuloalveolar glandular structures. The mammary pit is a depression in the epidermis where the lactiferous ducts converge. Near birth, the nipple is formed by evagination of the mammary pit. A congenitally inverted nipple is the result of failure of this normal process to occur.
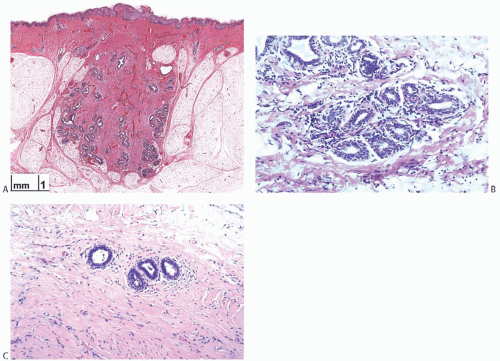
The earliest stages of fetal mammary gland formation appear to be independent of steroid hormones, whereas the actual development of the breast structure after the 15th week is influenced largely by testosterone. In the last weeks of gestation, the fetal breast is responsive to maternal and placental steroid hormones and prolactin, which induce secretory activity. This is manifested after birth by the secretion of colostrum and palpable enlargement of the breast bud. The secretory activity typically subsides and ceases during the first or second month after birth owing to disappearance of maternal hormones from the infant’s bloodstream. Thereafter, the gland shrinks and returns to an inactive state in which it is composed of lactiferous ducts that branch somewhat without progressive glandular differentiation, although lobular structures may persist (Fig. 1.1). Endocrine and paracrine factors involved in the “branching morphogenesis” of the mammary gland were reviewed in detail by Sternlicht.7
The protein product of the bcl-2 gene, which acts to inhibit apoptosis, is maximally expressed in the fetal breast.8 Immunohistochemical localization of bcl-2 has been detected in the basal epithelium of the developing breast bud and in the surrounding stroma of male and female breast tissues. Bcl-2 reactivity is lost soon after birth and it is absent from the epithelium of the normal adult breast. These observations suggest that upregulation of bcl-2 contributes to morphogenesis of the fetal breast by its inhibitory effect on apoptosis. Further normal breast development does not begin until puberty.
Premature Thelarche
Premature thelarche is the unilateral or bilateral appearance of a discoid subareolar thickening in girls with no other clinical evidence of sexual maturation prior to puberty.9 The condition may be related to environmental factors, an aberrant response to unusual hormone levels, or due to an activating mutation in the GNAS gene that codifies for a subunit of G-stimulating protein.10 Activation of the GNAS-1 gene in premature thelarche may occur in the absence of the other signs such as café au lait skin lesions and polyostotic fibrous dysplasia of bone associated with the McCune-Albright syndrome.10
The incidence of premature thelarche in white female infants and children up to 7 years old in the United States in 1980 was 20.8 per 100,000,11 and its prevalence, as reported in 2010 among 318 female children aged 12 to 48 months in a mid-western American hospital, was calculated to be 4.7%.12 The peak incidence was found between 12 and 17 months of age. The mean basal follicle-stimulating hormone (FSH) level in girls with premature thelarche is higher than that in normal controls and these girls have a greater response to gonadotrophin-releasing hormone.13 Patients with precocious puberty tend to have normal FSH levels and a normal response to luteinizing hormone-releasing hormone.14 Klein et al.15 reported that girls with premature thelarche had significantly higher levels of estradiol than do normal prepubertal girls.
The nodular breast tissue measuring 1.0 to 6.5 cm tends to regress slowly over the subsequent 6 months to 6 years, but in some instances, the hyperplastic breast bud persists until puberty.11 Curfman et al.12 reported that breast development persisted in 44% of infants and children with premature thelarche. Volta et al.16 reported that 60% of girls with premature thelarche that began before age 2 had complete regression prior to the onset of puberty. van Winter et al.11 reported that follow-up of women who had premature thelarche revealed no predisposition to breast carcinoma and a normal age of menarche. In another series, 14% of girls with premature thelarche developed precocious puberty,17 a circumstance more likely to occur if the onset of premature thelarche is after 2 years of age.13 Excision of the tissue that constitutes premature thelarche is contraindicated because this results in amastia.
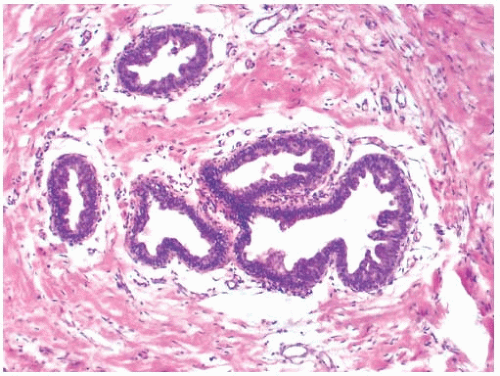
Histologically, the breast tissue in premature thelarche resembles gynecomastia because it is characterized by epithelial hyperplasia in the duct system with a solid and micropapillary configuration (Fig. 1.2). Growth and branching of the proliferating ducts results in an increased number of duct cross sections surrounded by moderately cellular stroma. Fine-needle aspiration (FNA) cytology reveals a background of myxoid stroma, bipolar stromal cells, and sparse sheets of benign ductal cells.18
Premature thelarche should be distinguished from prepubertal breast enlargement, which typically occurs as a
result of the accumulation of excess fat and connective tissue in the breast.
result of the accumulation of excess fat and connective tissue in the breast.
ADOLESCENT BREAST DEVELOPMENT
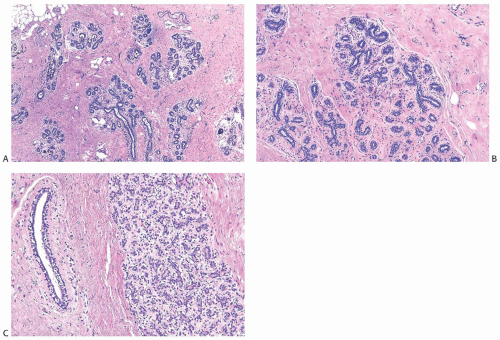
With the onset of cyclical estrogen and progesterone secretion at puberty, adolescent female breast development commences. Growth of ducts that elongate and acquire a thickened epithelium is dependent on estrogens.19 Differentiation of hormonally responsive, estrogen-dependent periductal stroma also occurs at this time. Growth hormone and glucocorticoids contribute to ductal growth. Terminal duct and lobular differentiation and growth during this period are enhanced primarily by insulin, progesterone, and growth hormone. The lobules are derived from solid masses of cells that form at the ends of terminal ducts. The greatest amount of breast glandular differentiation occurs during puberty, but the process continues for at least a decade and is enhanced by pregnancy7 (Fig. 1.3). The adolescent male breast consists of fibrofatty tissue and ducts lined by a thin layer of small cuboidal cells (Fig. 1.4).
GROSS ANATOMY OF THE ADULT BREAST
The mature breast has an eccentric configuration, with the long axis diagonally placed on the chest wall largely over the pectoralis major muscle and extending into the axilla as the tail of Spence. The peripheral anatomic boundaries of the breast are not precisely defined, except at the deep surface where the gland overlies the pectoralis fascia. Superficially the breast extends over portions of the serratus anterior muscle laterally, inferiorly over the external oblique muscle and superior rectus sheath, and medially to the sternum. The breast is extremely variable in size, shape, and weight (see Chapter 2).
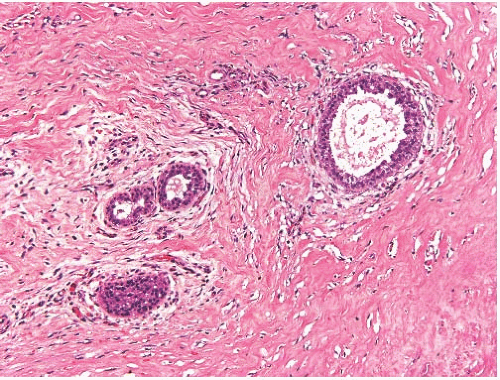 FIG. 1.4. Adolescent male breast. The thin layer of epithelium shows characteristic cellular crowding, and there is slight dilatation of a duct. |
Anatomically, the breast lies in a space within the superficial fascia, although microscopic extensions of glandular parenchyma sometimes traverse these boundaries. Superiorly, this layer is continuous with the cervical fascia, and inferiorly with the superficial abdominal fascia of Cooper. Fibrous strands extend from the dermis into the breast forming the suspensory ligaments of Cooper, which attach the skin and nipple to the breast. Cooper ligaments are more extensive in the upper portions of the breast. Distortion or contraction of the suspensory ligaments by parenchymal lesions may be manifested by skin dimpling or nipple retraction.
The deep membranous layer of the superficial fascia is separated from the fascia of the pectoralis major and serratus anterior muscles by the retromammary or submammary space that contains loose areolar tissue. Extensions of the membranous superficial fascia that traverse the retromammary space act as posterior suspensory ligaments. Microscopic extensions of glandular breast tissue may be found in conjunction with the posterior suspensory ligaments in the retromammary space and, rarely, in the underlying pectoral fascia. Neoplastic or inflammatory infiltration of the retromammary space is clinically associated with fixation of the breast to the chest wall.
The axillary fascia at the dome of the pyramidal axillary space is formed by an extension of the pectoralis major muscle. A fascial layer arising from the lower border of the pectoralis minor muscle joins an extension of the pectoralis major fascia to form the suspensory ligament of the axilla in continuity with the fascia of the latissimus dorsi muscle. An inconstant muscle band in this fascial plane is referred to as the suspensory muscle of the axilla. The fascial boundaries of the axilla provide important landmarks for the en bloc dissection of the axillary contents.
ARTERIAL SUPPLY AND VENOUS SYSTEM
The arterial circulation of the breast is derived from the internal thoracic, axillary, and intercostal arteries.20 There are many individual variations in the relative contributions of these vessels, and patterns of circulation are not necessarily symmetrical in the left and right breasts of an individual.21 Branches of the internal thoracic artery, which is also commonly referred to as internal mammary artery, provide the major source of arterial circulation in most individuals. These perforating branches traverse the thoracic wall at the sternal border in the first four intercostal spaces. The largest vessel usually lies in the second intercostal space. In about 30% of individuals, the axillary artery is of minor consequence, and in 50% there is little or no dependence on the intercostal arteries.22 Branches of the arterial circulation within the breast parenchyma do not specifically follow the major duct system.21
Venous drainage is more variable than the arterial supply, but it tends to follow the distribution of the arterial circulation.21 The superficial venous complex consists largely of transverse veins corresponding to branches of the internal thoracic artery. These vessels drain medially into the internal thoracic veins. A minor superficial venous system flows longitudinally toward the suprasternal notch to drain into superficial veins of the neck. Deep venous drainage is largely via perforating branches of the internal thoracic vein. Branches of the axillary vein also contribute to deep venous drainage and are especially prone to variable distribution. Tributaries of the intercostal veins provide a third route for venous drainage, with direct access to the vertebral veins and vertebral plexus.
LYMPHATIC DRAINAGE
A thorough description of the mammary lymphatics was first provided in 1786 by Cruikshank,23 who referred to lymphatic vessels as “absorbents.” He was able to identify the major routes of lymphatic flow from the breast as being along the course of the branches of the external thoracic and internal thoracic veins toward the axilla and internal mammary regions, respectively. Nearly a century later, Sappey24 employed mercury injection to demonstrate the lymphatic system of the lactating breast and observed drainage that appeared to flow from the parenchyma to the plexus of vessels in the subareolar region now referred to as the subareolar plexus of Sappey. This plexus serves as a pathway for cutaneous lymphatic drainage to the interlobular connective tissue of the breast and subsequently to the parenchymal lymphatic flow.
Various techniques have been used to study the pathways for intramammary lymphatic flow. These include dissection of static injected specimens, x-ray studies of Thorotrastinjected specimens,25 in vivo injection of colloidal gold,26 in vivo injection of a vital dye,27 and sentinel lymph node mapping with vital dyes and/or radioactive tracers. These investigations have resulted in conflicting observations regarding the pattern and amount of flow from different regions of the breast. In all likelihood, differing observations reflect limitations of the techniques employed and the intrinsic variability in lymphatic drainage between individuals.
Nonetheless, three dominant routes for mammary lymphatic drainage have been identified. The most important
of these is to the axilla that receives 75% or more of lymphatic flow into the axillary lymph nodes. Lymph nodes located in the interpectoral fascia constitute Rotter nodes. The most medial group of lymph nodes occurs at the apex of the axilla or level 3. The second pathway via internal lymphatics accounts for less than 25% of lymph flow. These vessels penetrate the pectoralis major and intercostal muscles to the internal thoracic mammary lymph nodes located along the sternal borders of the internal thoracic trunks. A third route for lymph drainage is via the posterior intercostal lymphatics to posterior intercostal lymph nodes in the chest where the ribs and vertebrae articulate. There are additional minor lymphatic channels draining to supraclavicular and infraclavicular lymph nodes as well as to intramammary lymph nodes.28
of these is to the axilla that receives 75% or more of lymphatic flow into the axillary lymph nodes. Lymph nodes located in the interpectoral fascia constitute Rotter nodes. The most medial group of lymph nodes occurs at the apex of the axilla or level 3. The second pathway via internal lymphatics accounts for less than 25% of lymph flow. These vessels penetrate the pectoralis major and intercostal muscles to the internal thoracic mammary lymph nodes located along the sternal borders of the internal thoracic trunks. A third route for lymph drainage is via the posterior intercostal lymphatics to posterior intercostal lymph nodes in the chest where the ribs and vertebrae articulate. There are additional minor lymphatic channels draining to supraclavicular and infraclavicular lymph nodes as well as to intramammary lymph nodes.28
Lymph drainage from any given region in the breast is not limited to one of the foregoing pathways.26,27 Nonetheless, correlation of patterns of lymph node metastases with the location of primary tumors in the breast suggests that preferential flow exists.28 For example, in the absence of axillary nodal metastases, the internal mammary lymph nodes are rarely affected, except when the primary tumor arises in the medial or central part of the breast.29 Conversely, tumors located in the upper outer quadrant are unlikely to metastasize only to internal mammary lymph nodes. Carcinomas that give rise to metastases in Rotter interpectoral lymph nodes are typically located in the upper outer and upper central regions.
Little lymphatic drainage traverses the deep fascia of the breast and the retromammary space. Although minute lymphatic channels have been identified in this fascia, minimal lymph from the mammary gland normally flows in this system. There is also no significant lymphatic flow to the contralateral internal mammary or axillary lymph nodes, but flow via these pathways may be augmented if ipsilateral drainage is obstructed as a result of therapy or by carcinoma.
Studies of sentinel lymph node mapping have provided further information about mammary lymphatic drainage in a physiologic setting and have suggested that a hierarchy exists in the anatomic and functional distribution of lymphatic flow to lymph nodes in the axilla. It is remarkable that this phenomenon can be demonstrated with seemingly equal specificity by injection of a tracer substance in the skin of the breast or in the parenchyma in the vicinity of a carcinoma. A thorough discussion of sentinel lymph node mapping can be found in Chapter 44.
FUNCTIONAL GROSS ANATOMY
The mature adult breast is composed of 15 to 25 grossly defined lobes of varying sizes, corresponding to parenchyma associated with each of the major lactiferous ducts that terminate in the nipple. No obvious landmarks defining the extent of individual lobes are found on inspection at operation or on dissection of the resected breast, and they are not evident in histologic sections. Three-dimensional reconstruction of the ductal system in the breast of a 19-year-old girl revealed that each duct drained an independent territory or “catchment.”30 The total volume drained by a duct and the length of the duct were highly variable. The existence of this functional lobar architecture provides an anatomical framework for treating some benign conditions by major duct excision and certain types of carcinoma by quadrantectomy.
Three-dimensional studies of 72 nipples in mastectomies performed for carcinoma have shown a variable number of nipple duct orifices (range 11 to 48, with a median of 27).31 In a detailed study of an anatomically normal breast obtained at autopsy, Going and Moffat31 found that one duct drained 23% of the breast, half of the breast was drained by three ducts, and that 75% was served by the six largest ducts. Three-dimensional reconstruction of a nipple by the same investigators using sequential histologic sections revealed three types of central nipple ducts. Seven of the 27 ducts had a lumen at the skin surface, although some were obstructed by keratin debris. A second group of ducts tapered to a minute lumen that ended in the epidermis in proximity to skin appendage glands. The superficial, narrowed segments of lactiferous ducts resembled the ducts of sweat glands. A third, relatively small group of open ducts appeared to be branches of the seven major patent ducts.
The nipple is covered by stratified squamous epithelium that is not pigmented in the prepubertal breast. Melanin pigmentation develops after menarche and increases during pregnancy, persisting to a variable degree thereafter. Sebaceous glands are present in the skin of the nipple. The areola surrounding the nipple is a ring of skin that undergoes pigmentary changes similar to those of the nipple. This specialized zone of mammary skin contains the glands of Montgomery that are modified sebaceous glands that open via ducts on the surface of the areola through the tubercles of Morgagni. The tubercles of Morgagni are especially visible during pregnancy around the base of the nipple. At this time, the areola appears to be “studded over and rendered unequal by the prominence of glandular follicles, which, varying in number from 12 to 20, project from the surface a sixteenth to an eighth of an inch.”32 The glands of Montgomery enlarge during pregnancy and contribute to a milk-like secretion that moistens the nipple and areola. These glands atrophy after menopause.
The functional glandular and ductal elements of the breast are embedded in fibrofatty tissue, which forms most of the mammary gland. The relative proportion of fat and collagenous stroma varies greatly among individuals and with age and is influenced by physiologic and hormonal factors. The combination of stromal and epithelial components is responsible for the radiographic appearance of breast structure in normal and pathologic states. Premenopausal women benefit from higher sensitivity of mammography performed during the first week of the menstrual cycle when the breast is least dense.33 The concept that mammographic pattern provides a guide to breast cancer risk, or for recommending preventative therapy, has not been validated; nonetheless, analysis of mammographic images was used to develop a classification scheme based on parenchymal density to predict cancer risk.34,35
Magnetic resonance imaging (MRI) provides a more precise method for discriminating between fatty and fibroglandular tissue in the breast without the attendant risk of radiation. By comparing images obtained with mammography and MRI, Lee et al.36 found a mean fat content of 42.5% (standard deviation [SD] ± 30.3%) in mammograms and 66.5% (SD ± 18%) in MRI images. The ranges of fat content obtained by mammography and MRI were 7.5% to 90% and 17% to 89%, respectively. The correlation coefficient for estimates of fat content obtained by both methods was 0.63, with the strongest correlation (r = 0.81) in postmenopausal women. Breast density determined by mammography is increased by exogenous hormone administration,37 with the greatest effect in postmenopausal women who received continuous combined estrogen-progesterone hormone replacement therapy. (HRT)38 Estrogen alone causes appreciably less increase in fibroglandular tissue when assessed by mammography39 or MRI




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree