(1)
Canberra, ACT, Australia
Summary
Patients are often apprehensive when undergoing root canal treatment and pain is often taken as synonymous with these procedures. Pain management involves correct diagnosis, appropriate dental treatment and adjunctive drug therapy where indicated. Endodontic pain will be of inflammatory origin and nonsteroidal anti-inflammatory drugs are the preferred choice for relief. Paracetamol provides effective analgesia with limited anti-inflammatory action. Effective pain management strategies are discussed for pre-, peri- and post-operative endodontic procedures.
Clinical Relevance
Effective pain management is a critical skill in order to attain patient satisfaction and trust when undergoing invasive root canal treatments. From a patients’ point of view, this aspect of treatment is often judged as more important than the challenging clinical practice of root canal itself. Several methods of pain relief are discussed, based on scientific evidence that will aim to provide adequate relief for patients experiencing pain and avoiding preconceived misconceptions that can be avoided by the use of modern pharmacology.
15.1 Overview of Analgesics, Anaesthetics, Anxiolytics and Glucocorticosteroids Used in Endodontics
Many patients frequently surmise root canal treatment as a painful procedure as a result of previous painful experiences and the perception that the treatment itself is painful [1]. These factors make pain management more challenging with the clinician not only trying to provide effective pharmacological relief but also removing preconceived ideas that provide barriers in the way of effective pain management strategies. The first signs of irreversible pulpal damage may be a patient presenting with a ‘hot’ tooth with spontaneous moderate to severe pain. The association of a painful experience is reinforced in the patients’ mind and proves to be a further challenge to the clinician due to difficulties in achieving adequate pulpal anaesthesia.
Effective strategies for the management of pain in the dental practice should follow the ‘3D principle’ of diagnosis, dental treatment and drugs. The first and most important step is to diagnose the condition correctly identifying the cause of the pain. Secondly appropriate dental treatment should then be undertaken to remove the cause providing rapid resolution of the symptoms. Finally drugs can be used as an adjunctive therapy to provide additional relief [2, 3].
Drugs available for the acute pain management can be classified into two major groups: the non-narcotic analgesics (e.g. nonsteroidal anti-inflammatory drugs (NSAIDs) and paracetamol) and opioids (narcotics). Aspirin, ibuprofen and paracetamol and the most common ‘over-the-counter’ non-narcotic analgesics are commonly used for managing dental pain. Three principle pharmacological approaches for the management of posttreatment endodontic pain include:
(a)
Drugs that block inflammatory mediators that sensitise or activate pulpal nociceptors (sensory neurones that respond to pain)
(b)
Drugs that block the propagation of impulses along the peripheral nerves
(c)
Drugs that block central mechanisms of pain perception and hyperalgesia
Pain management strategies during root canal treatment can be based on one or a combination of these mechanisms [4, 5].
Pre-emptive analgesia is the term used to describe the administration of an NSAID before the onset of pain (preventive measure) to suppress the release of inflammatory mediators (particularly prostaglandins), which contribute to the sensitisation of peripheral nociceptors [6, 7].
NSAIDs have been the traditional treatment for moderate endodontic pain management. Their mode of action is primarily through the inhibition of cyclooxygenase (COX) enzymes 1 and 2. COX-1 is expressed throughout the body and has a protective role in the stomach mucosa, kidney function and platelet action. COX-2 is induced by various endogenous compounds such as cytokines and endotoxins in inflammatory cells and is responsible for elevated prostaglandin production during inflammation [8–10]. Patients unable to tolerate NSAIDs include those suffering from gastrointestinal disorders (ulcers, ulcerative colitis), asthmatics or hypertensive patients (due to direct interactions with antihypertensive drugs or indirect effects due to the renal impairment) [11].
An alternative approach to treating a small proportion of patients who still report pain after administration of either an NSAID or paracetamol alone includes co-prescribing both drugs concurrently or combining either the NSAID or paracetamol with an opioid [12–14].
Corticosteroids (glucocorticosteroids) inhibit immune and inflammatory responses decreasing cytokine production, vasoactive and chemoattractive factors, secretion of lipolytic and proteolytic enzymes, extravasation of leucocytes to areas of tissue injury and fibrosis [15]. Several double-blind, random, prospective, placebo-controlled studies in endodontics have demonstrated the administration of adjunctive steroids being beneficial in reducing endodontic posttreatment pain [16–22]. Caution is urged when considering the use of steroids particularly in view of any medical history that may warrant contraindication [21].
Local anaesthesia is the mainstay of pain control techniques used in dentistry since its inception in 1859 by Albert Niemann who refined the coca extract to the pure alkaloid form and naming this drug ‘cocaine’. Today there is a spectrum of local anaesthetics that permit pain control to be tailored to the specific needs of the patient including short-, intermediate- and long-acting drugs [22]. Two proposed theories for the action of local anaesthetics include the non-specific membrane expansion theory and specific binding theory. In the former, swelling of the nerve membrane occurs from absorption of the lipophilic local anaesthetic resulting in inhibition of sodium into the cells, preventing nerve depolarisation and thus firing. The latter, now widely accepted, infers that specific binding receptors on the sodium channels are present for local anaesthetic agents to interact regulating influx of ions affecting depolarisation.
The primary local anaesthetics used in endodontics today are the amide-type local anaesthetics which have varying degrees of duration that can be selected according to specific patient and procedure needs (Tables 15.1 and 15.2) [23].
Table 15.1
Currently available amide-type dental local anaesthetic formulations
Local anaesthetic | % | Vasoconstrictor | Amount (mg) in 1.8 ml capsule |
|---|---|---|---|
Articaine | 4 | 1:100,000 adrenaline | 72 |
Bupivacaine | 0.5 | 1:200,000 adrenaline | 9 |
Lidocaine | 2 | 1:100,000 adrenaline | 36 |
2 | 1:50,000 adrenaline | 36 | |
2 | No vasoconstrictor | 36 | |
Mepivacaine | 2 | 1:20,000 levonordefrin | 36 |
3 | No vasoconstrictor | 54 | |
Prilocaine | 4 | 1:200,000 adrenaline | 72 |
4 | No vasoconstrictor | 72 |
Table 15.2
Currently available amide-type dental local anaesthetic formulations
Local anaesthetic Brand name | % | Vasoconstrictor | Expected duration | |
|---|---|---|---|---|
Pulpal (min) | Soft tissue (h) | |||
Articaine Ubistesin™ Septocaine® | 4 | 1:100,000 adrenaline | 60 | 3–5 |
Bupivacaine Marcaine® | 0.5 | 1:200,000 adrenaline | 90–180 | 3–12 |
Lidocaine Xylocaine® | 2 | 1:100,000 adrenaline | 60 | 3–5 |
2 | 1:50,000 adrenaline | 60 | 3–5 | |
2 | Plain no vasoconstrictor | 10 | 1–2 | |
Mepivacaine Mepivastesin™ | 2 | 1:20,000 levonordefrin | 60 | 3–5 |
3 | Plain no vasoconstrictor | 20–40 | 2–3 | |
Prilocaine Citanest® | 4 | 1:200,000 adrenaline | 60–90 | 3–8 |
4 | Plain no vasoconstrictor | 5–60 | 2–3 | |
The availability of a variety of local anaesthetic agents enables the clinician to select an anaesthetic that possesses properties such as time of onset and duration, haemostatic control and degree of cardiac side effects that are appropriate for each individual patient and for specific dental procedures. Lidocaine, mepivacaine and prilocaine, combined with a vasoconstrictor, provide reliable and profound pulpal anaesthesia for approximately 60 min (with a duration of soft-tissue anaesthesia lasting from 3 to 5 h) and are commonly used for endodontic procedures [24–30].
Articaine has become a very popular local anaesthetic in recent times with claims of superiority over existing anaesthetic agents in cases such as a difficult ‘hot pulps’ where extirpation requiring profound anaesthesia has not been achieved despite any clear evidence in the literature. Anecdotal evidence regarding a greater risk of long-lasting paraesthesia, especially the lingual nerve, when this drug is administered as a regional block, has also been reported [31–35].
Long-acting drugs such as bupivacaine have been shown to be useful in reducing or preventing post-operative discomfort in conjunction with orally administered nonsteroidal anti-inflammatory drugs [36, 37].
Anaesthetic failure after an inferior alveolar nerve block may be caused by several factors including collateral innervation [38–40], accessory innervation such as the mylohyoid nerve in mandibular posterior molar teeth [41, 42], inflammation-induced acidosis causing ‘ion trapping’ of local anaesthesia [43], pulpal sensitised nociceptors increasing anaesthetic resistance, central sensitisation [44] and psychological factors [45–47].
The use of topical anaesthesia has been investigated in numerous studies and despite no reports of significance influence on pain during either needle penetration or injection its use is still recommended. At the very least, the patient’s perception is one of the dentist trying to do everything possible to minimise pain during treatment [48, 49].
Primary anaesthetic techniques commonly used intra-orally in the maxilla include maxillary infiltrations. Additional techniques include the use of the posterior superior alveolar (PSA) nerve block, infraorbital nerve block, anterior superior alveolar (ASA) nerve block, anterior middle alveolar (AMA) nerve block and second division nerve blocks. In the mandible, the most common blocks include the conventional inferior dental nerve block, mental nerve block, long buccal nerve block and labial and lingual infiltrations. Alternative techniques commonly employed in the mandible include injection of solution at or near the condylar neck as in the Gow-Gates and Vazirani-Akinosi techniques [43, 50–62].
Supplemental anaesthetic techniques can be useful when conventional intra-oral anaesthesia has failed to achieve pulpal anaesthesia for endodontic procedures. These include intraosseous [63–67], intraligamentary (periodontal ligament) [68–72] and intra-pulpal injection techniques [73–75].
The traditional method of delivery of local anaesthetic solutions has been achieved using an aspirating needle and syringe. Although this method is proven to be effective, the technique itself is not completely pain-free [76]. Alternative local anaesthetic delivery systems and devices designed to minimise painful injections include needleless jet injector systems (INJEX Pharma, Berlin, Germany) [77, 78], vibrating supplementary devices (VibraJect system, ITL Dental, Irvine, CA, USA) and [79, 80], computer-controlled local anaesthetic devices (the Wand, Milestone Scientific, Livingston, NJM USA) [81–84].
Neurological complications following local anaesthesia have been reported in the literature including temporary facial nerve palsy and prolonged postinjection paraesthesia affecting the mental nerve, inferior dental nerve and lingual nerve. Less common nerve-related complications include ocular and extraocular symptoms including paralysis of extraocular muscles with associated diplopia and even amaurosis. Horner’s syndrome-type manifestations including enophthalmos, miosis and ptosis have been reported. Bleeding, muscle trismus, systemic complications including potential toxicity, loss of consciousness and generalised central nervous system depression, anaphylaxis-related allergy or a simple vasovagal syncope can occur after local anaesthetic administration. The clinician should be aware of the possible risks and methods employed to minimise such situations [85–89].
Oral sedation is a convenient technique for reducing anxiety experienced by some dental phobic patients. Other choices include sedation with nitrous oxide and oxygen, hypnosis, intravenous sedation and general anaesthesia. Benzodiazepines (diazepam, lorazepam, temazepam and triazolam) have been successfully used for oral sedation in dentistry by enhancing the action of the neurotransmitter γ-aminobutyric acid (GABA). This compound promotes an overall calming effect on the central nervous system influencing emotional reactions, memory, thinking and control of consciousness, muscle tone and co-ordination. Diazepam is the benzodiazepine of choice for oral dental sedation, which closely fulfils the criteria for an ideal sedation agent [90–94].
Pain is a complex subject; for patients, it is an unpleasant and emotional experience and may be associated with actual or potential tissue damage. In everyday clinical practice, practitioners face challenges in not only preventing or relieving dental pain but also ensuring that their treatment does not inflict pain [95]. The correct use of pre-emptive analgesics and anaesthetics armed with the knowledge of supplemental techniques should provide satisfactory pain relief for even those difficult to treat endodontic cases where patients present with a ‘hot tooth’. Occasionally oral sedation and adjunctive use of corticosteroids may be indicated to further reduce anxiety and pain in the endodontic office.
15.2 Analgesics
Pain relief as a result of endodontic intervention alone is rarely immediate but nevertheless the most effective strategy to reduce posttreatment pain. Posttreatment pain ranging from mild to severe may last up to 72 h in some cases requiring additional posttreatment analgesics. Several pharmacological strategies are available for ensuring effective pain management when treating the endodontic pain patient. When prescribing analgesics, patients should be instructed to take the medications at regular timely intervals (e.g. 6–8 hourly) ensuring that a more consistent blood level of the drug is achieved contributing to better pain relief. The two most common analgesics that can be prescribed include NSAIDs and paracetamol either separately or in combination. The decision to co-prescribe is based on the additive analgesic effect when these two drugs are taken together which is usually well tolerated by most patients when given over a short period of time. There may however be contraindications in the medical history that dictates which analgesics may best be tolerated altering the drug regime of choice.
The mechanism of action of most NSAIDs is believed to involve the inhibition of the cyclooxygenase enzyme (COX), thereby inhibiting the synthesis of prostaglandins. Pulpal inflammation and necrosis can lead to peri-radicular tissue injury that can activate phospholipase, which, in turn, releases arachidonic acid from cell membranes. The arachidonic acid in turn forms the substrate for the COX enzymes, which lead to the synthesis of various eicosanoids. Two COX isoforms exist, namely, COX-1 and COX-2. COX-1 is responsible for the production of prostaglandins with homeostatic functions in tissues such as the stomach, kidney and platelets, whilst COX-2 is responsible for the production of the prostaglandins involved in inflammation. The therapeutic effects of NSAIDs are attributable to the inhibition of COX-2.
Some patients may not be able to tolerate NSAIDs since inhibition of normal prostaglandin synthesis results in a number of adverse effects. These might include patients with gastrointestinal disorders (ulcers and ulcerative colitis), hypertension (including antihypertensive drug interactions or the renal effects of NSAIDs) and active asthmatics. For those patients who cannot tolerate NSAIDs, pretreatment with paracetamol is recommended.
15.3 Local Anaesthetic Solutions
Since ancient times, dentistry has unfortunately been associated with pain and discomfort – which has unfairly perpetuated to modern day. Prior to anaesthesia, a surgeon or dentist’s skill was equated with their speed of operation rather than the quality of their work. With the advent of anaesthesia, people have been able to undergo more extensive procedures, free of pain, anxiety and even memory. Despite these advances, however, people may still be apprehensive of dental procedures.
Local anaesthetics (LAs) are drugs that lead to a reversible block of transmission of impulses along neural pathways, both central and peripheral. By doing so, they effectively block the transmission of noxious pain stimuli along nerve fibres, thereby rendering an area of tissue insensate. Local anaesthetics exert their action by diffusing across nerve membranes, down a concentration gradient, and bind to the intracellular portion of voltage-gated sodium channels thus rendering it inactive. Local anaesthetic solutions exist in a solution consisting of an ionised and unionised fraction. It is the unionised fraction that is lipophilic and able to diffuse across the phospholipid bilayer of nerve cells and act at the voltage-gated sodium channels. Local anaesthetics are weak bases with a pKa >7.4 (the pKa of a drug is the pH at which 50 % is ionised and 50 % is unionised). As a result, at a physiological pH (7.4), most of the drug is in the ionised form.
The clinical correlation of this is that in infected, acidic or devitalised tissues, local anaesthetics may take a longer time to take affect (if at all) as majority of the drug is in the ionised form and cannot diffuse across the cellular membrane. Conversely, in order to have a faster speed of onset, some practitioners add the base sodium bicarbonate, thus increasing the unionised fraction of the drug. Other clinical characteristics include potency and duration of action. Potency is related to the lipid solubility of a drug and amount of drug available at a nerve (i.e. concentration). Duration of action is correlated with degree of protein binding. The greater the protein binding, the longer the duration of action.
Types and preparations
Local anaesthetics may be classified according to chemical structure. All local anaesthetics consist of an aromatic group and a tertiary amine. An intermediate bond connects the two parts. The intermediate bond may consist of either an ester or an amide. This gives rise to the classification of ester LAs and amide LAs. Examples of ester LAs include procaine (Novocaine) and cocaine. Examples of amide LAs include lidocaine (Xylocaine), prilocaine (Citanest), articaine (Septocaine), bupivacaine (Marcaine) and ropivacaine (Naropin).
Ester LAs are metabolised by degradation to para-aminobenzoic acid (PABA), which is highly antigenic and can result in allergic reactions. Once a PABA allergy occurs, patients may exhibit a cross reactivity to amide LAs that contain methylparaben as a preservative. Allergic reactions to amide LAs alone is extremely rare.
There are a wide variety of preparations of LAs available. In general, LAs are prepared as a hydrochloride salt, thus making them water-soluble. In addition, LAs for dental use often contain preservatives (such as methylparaben or sodium metabisulphite) and a fungicide.
Other common additives are vasoconstrictor drugs. These include adrenaline/epinephrine, felypressin or octapressin. Vasoconstrictor drugs are added to reduce the systemic absorption of the LA. Depending on the LA used, this potentially results in a prolongation of action. In addition, for the shorter-acting LA (e.g. lidocaine), a larger dose may be given before toxicity is reached. For example, the toxic dose of plain lidocaine is 4 mg/kg, whereas the toxic dose of lidocaine containing adrenaline/epinephrine is 7 mg/kg due to the decreased systemic absorption afforded by the vasoconstrictor. It must be noted however that for the commonly available long-acting LA (i.e. bupivacaine and ropivacaine), the addition of a vasoconstrictor does not change the toxic dose of the drug. With or without adrenaline/epinephrine, the toxic dose of bupivacaine is 2–2.5 mg/kg. This is due to the strong protein binding of the LA. The vasoconstrictor properties regress before LA detaches from the sodium channel.
There is no one LA that is suitable for all procedures. When choosing a LA, one needs to consider various factors including onset, offset, safety profile and cost. It has been suggested that for dental surgery, articaine comes closest to being the ideal LA owing to its fast onset, rapid metabolism, inactivation by organ- and non-organ-dependent pathways, availability and favourable safety profile.
Toxicity and management
Despite the example given in the preceding paragraph, LA toxicity cannot be only simplified into an mg/kg algorithm. Various different factors affect local anaesthetic toxicity, most notably site of injection (relative vascularity of site). For example, a patient may exhibit signs and symptoms of LA toxicity even if only 1–2 mL of LA is inadvertently injected into a major vessel, despite being well below the calculated toxic dose. Vascular tissue such as oral mucosa absorbs LA more readily than tissue with a less rich blood supply (e.g. subcutaneous tissue).
Nevertheless, toxic doses of local anaesthetic may be calculated based on lean body weight (see Tables 15.3 and 15.4).
Table 15.3
Toxic dose of local anaesthetic calculated on lean body weight
Lidocaine (plain) | 4 mg/kg |
Lidocaine (with adrenaline) | 7 mg/kg |
Prilocaine | 6 mg/kg |
Prilocaine (felypressin or octapressin) | 8 mg/kg |
Articaine | 7 mg/kg |
Bupivacaine | 2 mg/kg |
Ropivacaine | 3 mg/kg |
Table 15.4
Systemic effects of lidocaine
Plasma concentration (mcg/mL) | Effect |
|---|---|
1–5 | Analgesia |
5–10 | Light-headedness, tinnitus, circumoral numbness/metallic taste |
10–15 | Seizures |
15–25 | Coma/respiratory arrest |
>25 | Cardiac depression/arrest |
LA toxicity ultimately is directly related to plasma concentration of the drug and manifests itself in two major ways: cardiac toxicity and central nervous system (CNS) toxicity.
Central nervous system effects
LA drugs are able to cross the blood–brain barrier and act at the CNS. Typically at low plasma concentrations, CNS excitatory symptoms/signs predominate such as light-headedness, tinnitus, circumoral numbness, metallic taste in mouth and ultimately seizures. As the plasma concentration rises, or if there is a rapid rise in plasma concentration, then CNS depression (coma and respiratory depression) ensues. LA which are more lipid soluble (i.e. more potent such as bupivacaine) lead to CNS toxicity at much lower doses. Certain clinical states may make CNS more likely to occur. These include decreased protein binding, hypercarbia and systemic acidosis. Should the plasma concentration continue to rise, then cardiac toxicity may occur.
Cardiac effects
LA drugs can lead to potentially disastrous cardiac complications. In its mild form, LA drugs can lead to hypotension, arrhythmias and myocardial depression. Again, the more potent LAs such as bupivacaine and ropivacaine are the most sinister of these drugs as they can lead to fatal cardiac arrest (typically ventricular fibrillation, which is refractory to usual management) or complete heart block. Out of the long-acting LAs, ropivacaine is less cardiac toxic, as its affinity to the sodium channels on the cardiac myocyte is less. The exact mechanism of this is still not fully understood. The dose of LA causing CNS toxicity compared to dose causing cardiac toxicity gives rise to the concept of CC:CNS ratio. Lidocaine has a CC:CNS ratio of 7:1, whereas bupivacaine is 3:1 – thus implying that in regard to bupivacaine, there is only a very small margin of dose of local anaesthetic before cardiac toxicity occurs.
Management of local anaesthetic toxicity
The management of local anaesthetic toxicity is mainly supportive and is summarised in Table 15.5. In addition to the cardiac and neural manifestations of LA toxicity, prilocaine in doses >600 mg can lead to methaemoglominaemia. Methaemoglobinaemia results in impaired oxygen delivery to tissues and resultant tissue hypoxia. Treatment is with a reducing agent such as methylene blue.
Table 15.5
Management of local anaesthetic toxicity
Recognise LA toxicity, stop injection of the local anaesthetic solution and call for help |
If the patient is showing mild symptoms, only then reassure and continuously monitor until they improve. Preparations should be made in case signs of severe toxicity develop |
Administer oxygen and ensure ventilation is maintained and respiratory acidosis is avoided |
If conscious level is deteriorating or convulsions develop, the patient will require maintenance of airway; this may necessitate tracheal intubation. Hundred percent oxygen should be administered and adequate ventilation ensured |
If seizures develop, benzodiazepines can be administered in small incremental doses |
For cardiac arrest associated with local anaesthetic toxicity, cardiopulmonary resuscitation should be commenced using standard protocols. Cardiac arrhythmias may be treated with lipid emulsion therapy as per ALS guidelines. Consider Intralipid 20 % (1.5 mL/kg) over 1 min followed by infusion at 15 mL/kg/h. If no improvement, consider repeat bolus dose and increasing infusion rate. Cumulative Intralipid dose should not exceed 12 mL/kg |
Local anaesthesia mishaps
In addition to toxic effects affecting the central nervous system and heart, intra-oral local anaesthesia can result in complications.
Trismus
The inability to open the mouth can occur 2–5 days after a mandibular block has been administered. Inadvertent incorrect needle placement, accidental penetration of muscle or puncturing of blood vessels during administration of this block can lead to haematoma formation and subsequent fibrosis. Patients will often complain of marked trismus that can last several weeks if quite severe. Management includes reassurance and explanation of possible cause. Conservative management including use of hot packs and stretching exercises using wooden spatulas are usually sufficient for this condition. Rarely if the haematoma becomes infected, then surgical referral will be needed.
Facial nerve palsy (immediate)
Incorrect needle placement during either an inferior dental nerve block or posterior superior alveolar nerve block can result in a temporary facial palsy. Patients with peripheral facial nerve palsy will exhibit generalised weakness of the ipsilateral side of the face, inability to close the eyelids, obliteration of the nasolabial fold, drooping of the corner of the mouth and deviation of the mouth to the unaffected side. Management includes reassurance and explanation with emphasis that the weakness is temporary in nature. An eye-patch may be advisable to avoid ophthalmic damage.
Postinjection paraesthesia
Prolonged or permanent alteration of sensation along part or all of the distribution of either the maxillary or mandibular branches of the trigeminal nerve can occur following administration of local anaesthetic. These altered sensations can be categorised as anaesthesia (total absence of sensation), paraesthesia (abnormal sensations such as ‘pins and needles’) and dysesthesia (spontaneous or mechanically evoked painful neuropathy). They can be attributed to a number of theories including direct trauma from the needle, intra-neural blood vessel trauma and intra-neural haematoma formation and neurotoxicity directly related to the local anaesthetic used. During inferior alveolar nerve block administration, the patient may on occasion experience an immediate electric shock sensation. This is thought to be due to the needle contacting the nerve trunk, which can potentially cause direct damage to the nerve and long-lasting altered sensation of various durations. The practitioner is advised to stop the administration of the anaesthetic solution, and repositioning of the needle is recommended a few millimetres away. The patient should be warned of the potential temporary paraesthesia that can develop which should be transient in nature but may take several weeks to fully recover. Management of anaesthesia, paraesthesia and dysesthesia will require sensory testing of the affected distribution, reassurance and follow-up. A surgical referral may be required in some patients to ensure prompt surgical intervention is carried out if deemed necessary.
15.4 Topical Anaesthesia
The use and efficacy of topical anaesthesia prior to needle insertion and injection of local anaesthesia has been highly variable. Nevertheless, its use is recommended prior to all intra-oral dental anaesthesia techniques to rest assure the patient that the clinician is trying everything possible to minimise any pain during treatment. Topical anaesthetic gel formulations should be placed over the mucosa at least 30 s to 1 min before the injection (see Fig. 15.1).
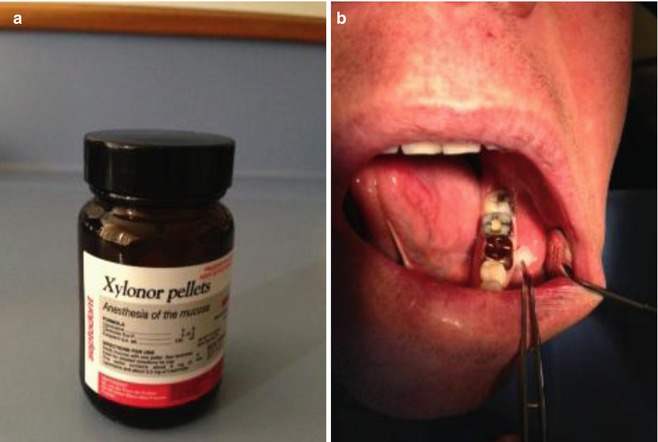

Fig. 15.1
Clinical photographs demonstrating (a) Xylonor topical anaesthetic pellets. One pellet contains 100 mg of lignocaine-based solution. Useful for topical anaesthesia of the mucosa prior to injection. (b) The mucosa is dried and then the topical anaesthetic pellet is massaged and left in situ for 30 s
15.5 Maxillary Infiltration and Blocks
The pulpal sensory fibres of the maxillary teeth are primarily carried in the anterior, middle and posterior superior alveolar nerves, which also supply the buccal soft tissues. Most problems with maxillary anaesthesia can be attributed to individual variances of normal anatomical nerve pathways within the maxillary bone with accessory pulpal innervation fibres found in the palatal innervation supplied by the nasopalatine and greater palatine nerves. The administration of palatal anaesthesia is often described as a painful experience by the patient, and direct palatal infiltration is difficult to administer without significant pain or discomfort since there is little tissue space at these sites between the mucosa and the underlying. Using careful application of topical anaesthesia, distraction techniques and ensuring slow delivery of anaesthetic solution should allow for very little to no patient discomfort. Alternative injections using articaine in the buccal vestibule alone may prove sufficient, avoiding the palatal injection. New computer-controlled anaesthetic delivery systems (CCLAD) are particularly proficient at eliminating or at the very least minimising discomfort when giving injections in the palate.
Nasopalatine nerve block
Palatal anterior superior alveolar nerve block (PASA)
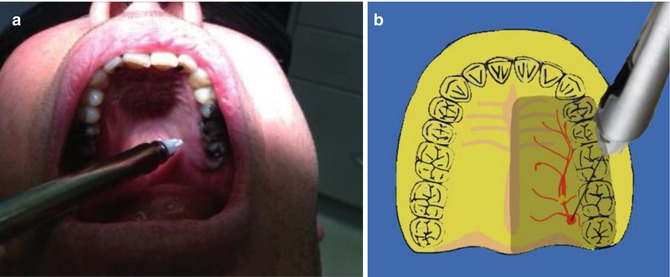
The needle is placed just lateral to the incisive papilla (Fig. 15.2). Once the needle has penetrated the mucosa, initial deposition of anaesthetic solution is injected over 10 s. Following this the needle is reoriented more vertically to enter the nasopalatine canal a distance of 0.5–1 cm. Following negative aspiration, the remaining solution within the cartridge is deposited. The injection will provide anaesthesia of all six maxillary anterior teeth (canine to canine) with a single injection including soft tissue overlying the anterior palate (nasopalatine nerve distribution) and to a lesser extent the labial gingivae.
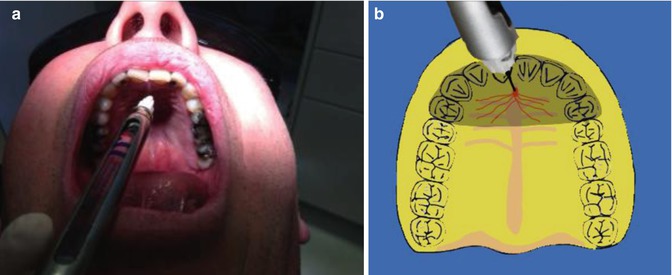

Fig. 15.2
(a, b) Clinical photographs and diagrams demonstrating the nasopalatine block injection. The entrance to the nasopalatine foramen is at the incisive papilla, which may be visualised posterior to the maxillary central incisors. The needle tip should contact the soft tissue at the lateral aspect of the incisive papilla with a depth of penetration of less than 5 mm. After aspiration anaesthetic solution should be deposited at a very slow rate to minimise discomfort for the patient
Greater palatine nerve block
This block is used to anaesthetise the palatal soft tissue of the teeth posterior to the maxillary canine and corresponding alveolar bone in the palate. The needle is inserted approximately 1 cm medial to the 1st/2nd maxillary molar on the hard palate. The needle is advanced into the greater palatine foramen to a depth of less than 10 mm before injecting anaesthetic solution (Fig. 15.3).