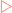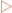chapter 17 Anabolic metabolism
 Glycogen synthase and glycogen phosphorylase are controlled by the same mechanism to balance glycogen synthesis and breakdown.
Glycogen synthase and glycogen phosphorylase are controlled by the same mechanism to balance glycogen synthesis and breakdown. Gluconeogenesis is the pathway that can synthesise glucose from alternative sources such as amino acids.
Gluconeogenesis is the pathway that can synthesise glucose from alternative sources such as amino acids. The rates of glycolysis and gluconeogenesis are coordinated by the level of fructose-2,6-bisphosphate.
The rates of glycolysis and gluconeogenesis are coordinated by the level of fructose-2,6-bisphosphate.Anabolism
This chapter covers some of the major anabolic path ways of the cell involved in the synthesis of amino acids and nucleotides, glycogen, lipids and glucose. The use of nucleotides in the synthesis of DNA and amino acids in the synthesis of proteins is discussed in Chapter 20.
Amino acid synthesis
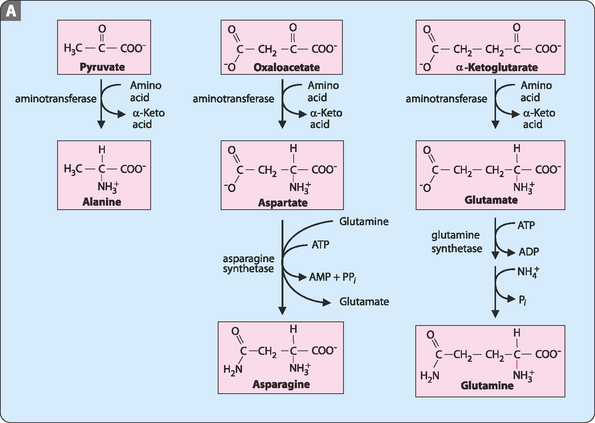
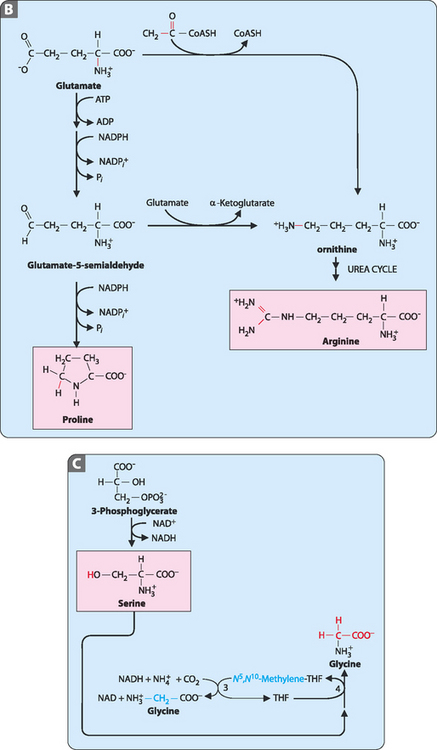
As seen in Chapter 15, of the 20 amino acids required for the synthesis of proteins 9 are considered non-essential. These amino acids are synthesised from intermediates of either the TCA cycle (pyruvate, oxaloacetate, α-ketoglutarate) or glycolysis (3-phosphoglycerate). Completion of the synthesis of many of these amino acids requires a number of enzymatic reactions and, often, previously synthesised amino acids are substrates. The synthetic pathways are shown in Figure 17-1.
Nucleotide synthesis
One molecule of key importance in the synthesis of nucleotides is tetrahydrofolate (THF; Fig 17-2). This molecule is derived from dietary folate (vitamin B9) and is used to transfer single-carbon groups such as methyl (N5,N10-methylene-THF) or formyl (N10-formyl-THF).
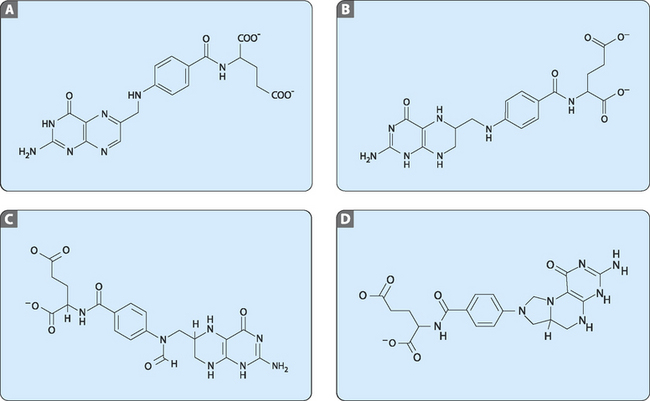
FIGURE 17-2 The structures of A, folate; B, tetrahydrofolate (THF); C, N10-formyl-THF and D, N5,N10-methylene-THF.
The backbone of nucleotides
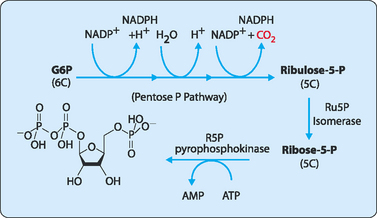
The key first step in the synthesis of any of the nucleotides is the formation of the ribose, which will then become the ‘backbone’ of any DNA or RNA molecule. The ribose is initially made in an activated form as the phosphorylated monosaccharide, phosphoribosyl-1-pyrophosphate (PRPP). The glycolytic intermediate, glucose-6-phosphate, is used by the pentose phosphate pathway to give ribulose-5-phosphate, which is then converted to ribose-5-phosphate before being further phosphorylated to give PRPP (Fig 17-3).
Synthesis of purines
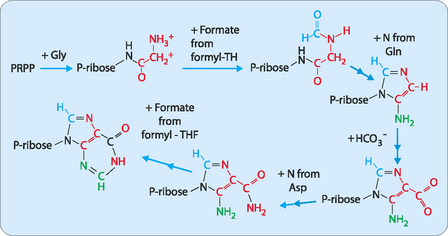
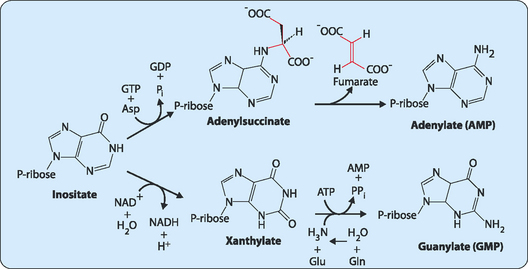
The synthesis of the purine bases (A and G) is carried out by the consecutive addition of each of the components of the purine base onto a molecule of ribose-5-phosphate (Fig 17-4). Each part of the purine is derived from amino acids (glycine, glutamine or aspartate), N10-formyl-tetrahydrofolate (THF) and bicarbonate. This pathway forms inosine monophosphate which is then further modified to give either adenosine or guanosine monophosphate (Fig 17-5).
Synthesis of pyrimidines
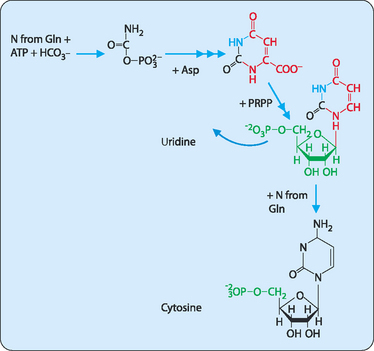
The synthesis of pyrimidines differs from that of purines in that the base is synthesised before being added to PRPP (Fig 17-6). The initial step in pyrimidine synthesis is the formation of carbamoyl phosphate from glutamine, ATP and bicarbonate in the cytosol. Carbamoyl phosphate is also created in the initial stages of the urea cycle; however, this occurs in the mitochondrion, not the cytosol. Aspartate is then used to form the rest of the pyrimidine base before it is added to PRPP to give uridine monophosphate (UMP). UMP can then be phosphorylated to yield UDP, then UTP, which is then aminated to CTP using glutamate as the amino donor.
The final steps
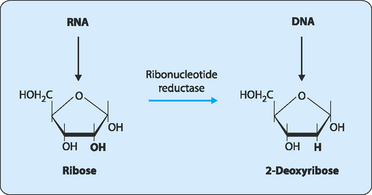
The deoxy forms of the nucleotides are synthesised by the enzyme ribonucleotide reductase using GDP, ADP, CDP or UDP as substrate (Fig 17-7).
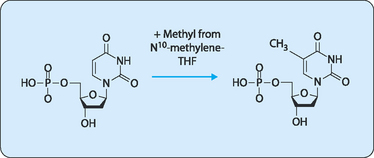
The final piece of the puzzle is the methylation of dUTP to give dTTP by the enzyme thymidylate synthase, which uses N5,N10-methylene-THF to provide the methyl group (Fig 17-8).