Cardiac
Cardiac adverse effects are related to amiodarone’s effects on the action potential and include bradyarrhythmias, worsening of conduction defects, and ventricular arrhythmias.37,38 In patients with an ICD, ventricular tachycardias may occur at a lower rate than the detection rate, and arrhythmias may go unrecognized. The defibrillation threshold may be increased, and ICD treatments may be ineffective. Hypotension may be a side effect especially with the IV form and is thought to be related to its α-blocking properties.
Extracardiac
Amiodarone has significant extracardiac effects on multiple organ systems. Because of its high iodine content and close structural association to thyroxine and effects on thyroid metabolism, hypothyroidism may be seen in up to a third of patients.39 Thyroid screening is indicated at baseline and 6 months after starting therapy as it usually takes a few months for hormonal levels to equilibrate.25 Hypothyroidism is diagnosed with a high TSH level that reverses after discontinuation of the drug. If hypothyroidism persists, possibly due to intrinsic defects in thyroid synthesis, thyroid supplements are required. Hyperthyroidism is a much less frequent problem but may occur any time during therapy. The typical clinical symptoms of hyperthyroidism may be masked due to the β-blocking effect of the drug. Treatment with antithyroid agents may be needed in addition to discontinuation of the drug.40
Pulmonary toxicity can occur in up to 5% to 20% of patients on chronic doses and can manifest as pulmonary fibrosis, chronic interstitial pneumonitis, organizing pneumonia, bronchiolitis obliterans, or ARDS.41–44 Toxicity involving the lung is usually related to the cumulative dose but may occur any time during the course of therapy, and data supports involvement at both low and higher dose ranges.45 The usual presentation is an initial cough, followed by progressive dyspnea. A high index of suspicion is required as these may be confused with heart failure symptoms. Pulmonary function tests show restrictive lung disease and a decreased diffusion capacity. Though this is a sensitive test, the specificity is limited and therefore not suggested as a routine monitoring tool.25 Management includes discontinuing the drug and initiation of steroids. Gastrointestinal symptoms are usually dose-related, though long-term effects on the liver may be seen with chronic accumulation. An increase in transaminase levels may be seen in 10% to 20% of patients, though hepatitis is relatively rare. Corneal microdeposits may be seen in 50% to 100% of patients with prolonged therapy. Although these do not affect vision, the deposits can cause cysts and abscesses, but usually reverse with drug discontinuation. Another reversible side effect, with long term use, is blue gray discoloration of the skin seen in up to 10% of patients.46 Skin photosensitivity with exposure to sunlight may be seen as an acute or chronic effect. Other side effects include gastrointestinal upset and neurological problems such as headache, proximal muscle weakness, peripheral neuropathy, and ataxia.24 Due to these numerous side effects, under two-thirds of patients remain on therapy 5 years after treatment.47
DRUG INTERACTIONS
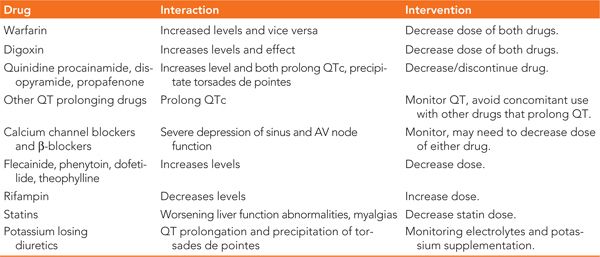
Amiodarone has multiple drug interactions especially with drugs that are metabolized in the liver or highly protein bound.48 The interactions are summarized in Table 32-2.
TABLE 32-2 Drug Interactions with Amiodarone
PREGNANCY AND PEDIATRIC PATIENTS
Amiodarone can cross the placental barrier due to its high lipid solubility and can affect the developing fetus. It should therefore be avoided in pregnancy.49,50 Despite not being adequately studied in children, the IV form is frequently used in atrial and ventricular tachyarrhythmias after congenital heart surgery. Due to its side effects, it is less commonly used for long-term maintenance therapy.
SUMMARY
In summary, amiodarone is the most effective antiarrhythmic agent for the treatment of AF and ventricular tachycardias. Amiodarone has a low propensity for proarrhythmia and can be initiated as an outpatient drug and safely used in most patients with structural heart disease. Due to its potential to cause serious end-organ toxicity, amiodarone should be reserved to treat patients who have not been successfully treated with other antiarrhythmic agents. Close monitoring is essential as side effects can manifest at any time during the course of therapy.
REFERENCES
1. Deltour G, Binon F, Tondeur R, et al. Studies in the benzofuran series. VI. Coronary-dilating activity of alkylated and aminoalkylated derivatives of 3-benzoylbenzofuran. Arch Int Pharmacodyn Ther. 1962;139:247-254.
2. Rosenbaum MB, Chiale PA, Halpern MS, et al. Clinical efficacy of amiodarone as an antiarrhythmic agent. Am J Cardiol. 1976;38(7):934-944.
3. Mason JW. Drug therapy: amiodarone. N Engl J Med. 1987;316(8):455-466..
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


