Chapter 39 Hallucinogenic, allergenic, teratogenic and other toxic plants
HALLUCINOGENS
FUNGI
The Amanitas
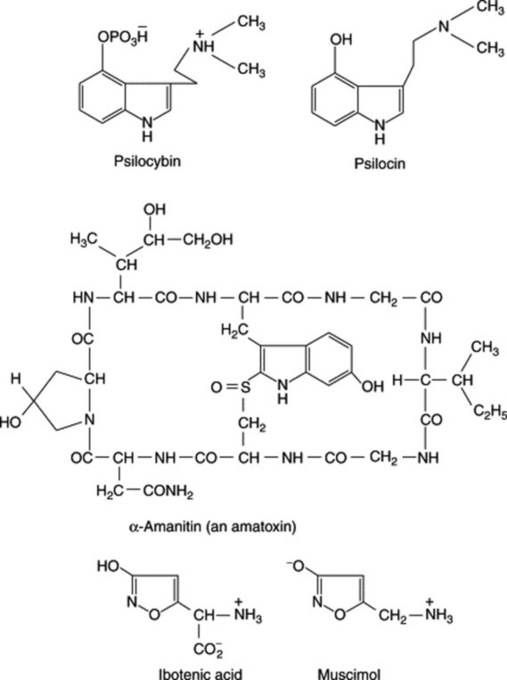
A number of Amanita species, in addition to promoting hallucinogenic effects, are extremely toxic. The appearance of the serious symptoms is considerably delayed (particularly with amatoxins, formula Fig. 39.1) after ingestion, by which time effective treatment becomes difficult. Three classes of toxins are recognized in the genus—tryptamines (e.g. bufotenine), cyclic peptides (phallotoxins and amatoxins) and isoxazole alkaloids (e.g. ibotenic acid, formula Fig. 39.1). The three classes of compound appear to be restricted to certain specific sections of the genus.
Hallucinogenic Mexican mushrooms
A number of small toadstools—particularly species of Psilocybe (P. mexicana), Conocybe (C. cyanopus) and Stropharia—constitute the Mexican hallucinogenic mushrooms (teonanacatl, ‘flesh of the gods’, much revered by the Aztecs). The onset of symptoms after ingestion of the fungi is rapid, and includes inability to concentrate and the occurrence of hallucinations. The active constituents are the tryptamine derivatives psilocybin and psilocin, compounds related to serotonin. These compounds are also found in similar toadstools (e.g. Psilocybe and Paneolus, Copelandia, Gymnopilus, Inocybe, Panneolina, Pluteus and Stropharia spp.) which are found in temperate regions. In Britain the ‘liberty cap’, Psilocybe semilanceata, a small toadstool common on lawns and parkland, and in Australia P. subaeruginosa both contain psilocybin. What is claimed to be the highest proportion of psilocin contained in any mushroom (3.3%, dry weight) was reported in Psilocybe cubensis; for a review of the mushroom alkaloids (567 refs), see R. Autkowiak et al., Alkaloids, 1991, 40, 189); for the concise large-scale synthesis of psilocin and psilocybin, see O. Shirota et al., J. Nat. Prod., 2003, 66, 885.
PEYOTE
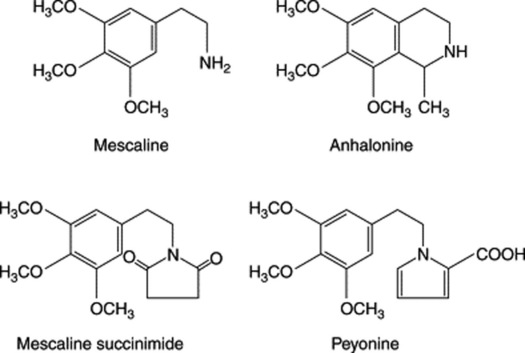
Certain cacti are of pharmaceutical and pharmacological interest, as they contain protoalkaloids, some of which have marked hallucinogenic properties. One of these is the cactus Lophophora williamsii which has long been used by Mexican Indians. It is known as peyotl, anhalonium or mescal buttons. The latter name is derived from the cactus stems, which are cut into slices about 20–50 mm in diameter. An interesting report (H. R. El-Seedi et al., J. Ethnopharm., 2005, 101, 238) sheds more light on its possible use by native N. Americans some 5700 years ago: two specimens from the Witte Museum in San Antonio were radiocarbon-dated to 3780–3600 BCE and, on analysis (TLC and GC-MS), gave alkaloids (2%) in which mescaline was identified, making these the oldest examples of a plant drug to yield a major bioactive compound. The chief active constituent is the alkaloid mescaline. The chemistry dates back to 1888, in which year Lewin isolated anhalonine, a crystalline tetrahydroisoquinoline alkaloid. By 1973 some 56 alkaloids had been characterized from the cactus and these could be classified as (1) mono-, di- and trioxygenated phenethylamines and their amides; (2) tetrahydroisoquinoline alkaloids and their amides; (3) phenethylamine conjugates with Kreb’s cycle acids; (4) pyrrole derivates. Examples of these groups are given in Fig. 39.2. The alkaloids can arise in the plant from dopamine, and grafting experiments involving Trichocereus pachanoi (‘San Pedro’) (a mescaline-producing species) and T. spachianus (no mescaline) have indicated that biosynthesis of the hallucinogen is confined to the aerial parts.
Hemp products
Three main type of narcotic product are produced.
Microscopical characters
The resin is secreted by numerous glandular hairs, 130–250 μm long (see Figs 39.3; 42.5). The head is usually eight-celled and the pedicel multiseriate or unicellular. To differentiate these from similar trichomes (e.g. those of the Labiatae) specificstains can be used. These include Fast Blue Salt B and, as described by Corrigan and Lynch in 1978, a reagent consisting of vanillin in ethanolic sulphuric acid which stains the cannabis glands a deep reddish-purple. It is possible to analyse individual trichomes by GLC by which means it has been shown that the glands represent a dynamic system in the cannabinoid synthetic activity of the plant. In addition, sessile glands (Fig. 39.3), abundant conical, curved, unicellular hairs, are also found, many having cystoliths of calcium carbonate in their enlarged bases (see Figs 39.3; 42.3); however, these cystolith hairs are not confined solely to the genus Cannabis. Cluster crystals of calcium oxalate are abundant, particularly in the bracteoles.
Constituents
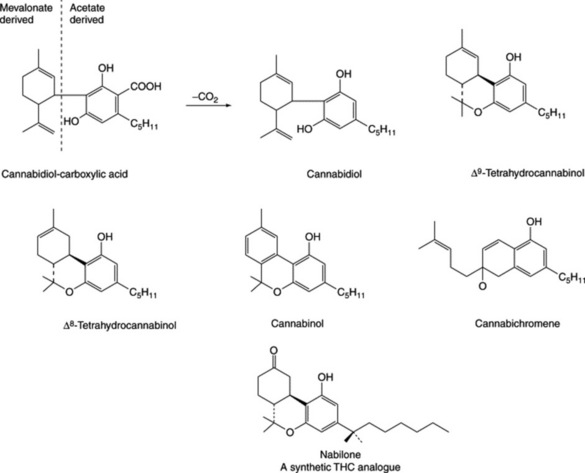
The narcotic resin is a brown, amorphous semisolid; soluble in alcohol, ether and carbon disulphide. It contains over 60 compounds (cannabinoids) all composed of an aromatic portion (C11 or C12), theoretically derivable from six acetate units, and an isoprenoid component (C10). They appear to form a natural group of C21 terpenophenolics of unique occurrence. Some principal components are cannabinol, tetrahydrocannabinol (THC), cannabidiol (CBD), cannabidiol-carboxylic acid, cannabigerol and cannabichromene (Fig. 39.4). Cannabinodiol is the aromatic analogue of cannabidiol. Cannabigerol precedes Δ9-THC in the biosynthetic pathway and is incorporated, by the plant, into the latter and other neutral cannabinoids. The identification of phloroglucinol β-D-glucoside in the shoot laticifer exudate of C. sativa, and phloroglucinol as a prominent component, and the only phenol, in the glandular trichomes suggests that it may have an important role in the in vivo enzymatically regulated biosynthesis of the cannabinoids (C. T. Hammond and P. G. Mahlberg, Phytochemistry, 1994, 37, 755).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree