Chapter 26 Alkaloids
INTRODUCTION
DISTRIBUTION
True alkaloids are of rare occurrence in lower plants. In the fungi the lysergic acid derivatives and the sulphur-containing alkaloids, e.g. the gliotoxins, are the best known. Among the pteridophytes and gymnosperms the lycopodium, ephedra and Taxus alkaloids have medicinal interest. Alkaloid distribution in the angiosperms is uneven. The dicotyledon orders Salicales, Fagales, Cucurbitales and Oleales at present appear to be alkaloid-free. Alkaloids are commonly found in the orders Centrospermae (Chenopodiaceae), Magnoliales (Lauraceae, Magnoliaceae), Ranunculales (Berberidaceae, Menispermaceae, Ranunculaceae), Papaverales (Papaveraceae, Fumariaceae), Rosales (Leguminosae, subfamily Papilionaceae), Rutales (Rutaceae), Gentiales (Apocynaceae, Loganiaceae, Rubiaceae), Tubiflorae (Boraginaceae, Convolvulaceae, Solanaceae) and Campanulales (Campanulaceae, sub-family Lobelioideae; Compositae, subfamily Senecioneae).
STRUCTURE AND CLASSIFICATION
Alkaloids show great variety in their botanical and biochemical origin, in chemical structure and in pharmacological action. Consequently, many different systems of classification are possible. In the arrangement of the well-known drugs which follow later in the chapter, the phytochemical arrangement introduced in the eleventh edition of this book and based on the origin of the alkaloids in relation to the common amino acids has been used. For practical purposes it is useful, therefore, to maintain the well-established classifications based on chemical structures, Fig. 26.1, and Table 26.1 closely follows that used by a number of authors. There are two broad divisions:
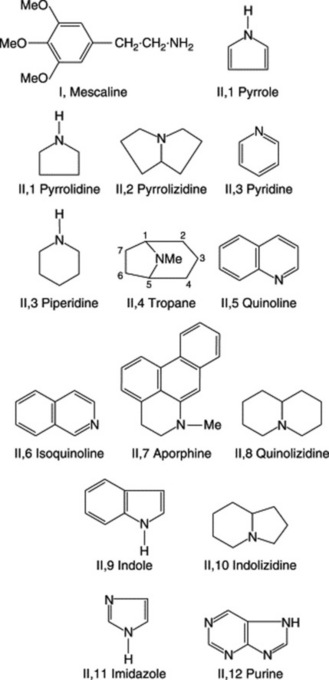
Fig. 26.1 Skeletal structures of alkaloids found in medicinal plants. (Numbers refer to location in Table 26.1).
Table 26.1 Types of alkaloid and their occurrence.
| I. Non-heterocyclic alkaloids | |
| Hordenine or N-methyltyramine | In germinating barley, Hordeum distochon |
| Mescaline, related to tryptamine (see formula) | Lophophora williamsii (Cactaceae) |
| Ephedrine | Ephedra spp. (Ephedraceae) |
| Colchicine (tropolone nucleus with nitrogen in side-chain) | Colchicum spp. and related genera (Liliaceae) |
| Erythromycin (an antibiotic) | Streptomyces erythreus (Bacteriophyta, Actinomycetales) |
| Jurubin (steroid with 3-amino group) | Solanum paniculatum (Solanaceae) |
| Pachysandrine A (steroid with N-containing C-17 side-chain) | Pachysandra terminalis (Buxaceae) |
| Taxol (a modified diterpene pseudo alkaloid) | Taxus brevifolia (Taxaceae) |
| II. Heterocyclic alkaloids | |
| 1. Pyrrole and pyrrolidine | |
| Hygrines | Coca spp. (Erythroxylaceae); often associated with tropane alkaloids of the Solanaceae |
| Stachydrine | Stachys tuberifera (Labiatae), soya bean and other Leguminosae |
| 2. Pyrrolizidine | |
| Symphitine, echimidine | Symphytum spp. |
| Senecionine, seneciphylline, etc. | Senecio spp. |
| 3. Pyridine and piperidine | |
| Trigonelline | Fenugreek (Leguminosae), strophanthus (Apocynaceae), coffee (Rubiaceae) |
| Coniine | Conium maculatum (Umbelliferae) |
| Arecoline | Areca catechu (Palmae) |
| Lobeline | Lobelia spp. (Lobeliaceae) |
| Pelletierine | Punica granatum, the pomegranate (Punicaceae) |
| Nicotine (pyridine + pyrrolidine) | Nicotiana tabacum and other spp. (Solanaceae) |
| Anabasine | Nicotiana glauca; Anabasis aphylla (Chenopodiaceae) |
| Piperine | Piper spp. (Piperaceae) |
| Ricinine | Ricinus communis (Euphorbiaceae) |
| 4. Tropane (piperidine/N-methyl-pyrrolidine) | |
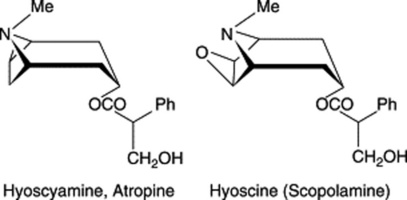
| Hyoscyamine, atropine, hyoscine, meteloidine, etc. | Species of Atropa, Datura, Hyoscyamus, Duboisia, Mandragora and Scopolia (Solanaceae) |
| Calystegines | Convolvulus spp., Ipomoea polpha (Convolvulaceae), some solanaceous spp., Morus spp. (Moraceae) |
| Cocaine | Coca spp. (Erythroxylaceae) |
| Pesudo-pelletierine | Punica granatum (Punicaceae) |
| 5. Quinoline | |
| Quinine, quinidine, cinchonine, cinchonidine | Cinchona spp. (Rubiaceae), Remijia spp. (Rubiaceae) |
| Cusparine | Angostura or cusparia bark, Galipea officinalis (Rutaceae) |
| 6. Isoquinoline | |
| Papaverine, narceine, narcotine | Papaver somniferum (Papaveraceae) |
| Corydaline | Corydalis and Dicentra spp. (Fumariaceae) |
| Hydrastine, berberine | Numerous genera of the Berberidaceae, Ranunculaceae and Papaveraceae |
| Emetine, cephaeline | Cephaelis spp. (Rubiaceae) |
| Tubocurarine | Curare obtained from plants of Menispermaceae |
| Morphine, codeine | Papaver somniferum (Papaveraceae) |
| Erythraline | Erythrina spp. (Leguminosae) |
| Galanthamine | Leucojum aestivum (Amaryllidaceae) |
| 7. Aporphine (reduced isoquinoline/naphthalene) | |
| Boldine | Peumus boldus (Monimiaceae) |
| 8. Quinolizidine | |
| Sparteine, cytisine, lupanine, laburnine | Sometimes called ‘the lupin alkaloids’. Occur particularly in the Leguminosae, subfamily Papilionaceae, e.g. broom. Cytisus scoparius; dyer’s broom, Genista tinctoria; Laburnum and Lupinus spp. |
| 9. Indole or benzopyrrole | |
| Ergometrine, ergotamine | Claviceps spp. (Hypocreaceae) |
| Lysergic acid amide, clavine alkaloids | Rivea corymbosa, Ipomoea violacea (Convolvulaceae) |
| Physostigmine | Physostigma venenosum (Leguminosae) |
| Ajmaline, serpentine, reserpine | Rauwolfia spp. (Apocynaceae) |
| Yohimbine, aspidospermine | Aspidosperma spp. (Apocynaceae) |
| Vinblastine, vincristine | Catharanthus roseus (Apocynaceae) |
| Calabash curare alkaloids | Strychnos spp. (Loganiaceae) |
| Strychnine, brucine | Strychnos spp. (Loganiaceae) |
| 10. Indolizidine | |
| Castanospermine | Castanospermum australe (Leguminosae), Alexa spp. (Leguminosae) |
| Swainsonine | Swainsona spp. (Leguminosae), Loco plants (Leguminosae) |
| 11. Imidazole or glyoxaline | |
| Pilocarpine | Pilocarpus spp. (Rutaceae) |
| 12. Purine (pyrimidine/imidazole) | |
| Caffeine | Tea (Ternstroemiaceae), coffee (Rubiaceae), maté (Aquifoliaceae), guarana (Sapindaceae), cola nuts (Sterculiaceae) |
| Theobromine | Cocoa (Sterculiaceae) |
| 13. Steroidal (some combined as glycosides) | |
| Solanidine (glycoside = solanine) | Shoots of potato (Solanaceae), etc. |
| Veratrum alkamine esters and their glycosides | Veratrum spp. and Schoenocaulon spp. (Liliaceae) |
| Conessine | Holarrhena antidysenterica (Apocynaceae) |
| Funtumine | Funtumia elastica (Apocynaceae) |
| 14. Terpenoid | |
| Aconitine, atisine, lyctonine, etc. | Aconitum and Delphinium spp. (Ranunculaceae) |
The nitrogen of alkaloids
Alkaloids, taken in their broadest sense, may have a nitrogen atom which is primary (mescaline), secondary (ephedrine), tertiary (atropine) or quaternary (one of the N atoms of tubocurarine), and this factor affects the derivatives of the alkaloid which can be prepared and the isolation procedures. In the plant, alkaloids may exist in the free state, as salts or as amine or alkaloid N-oxides.
Alkaloid N-oxides
N-oxidation products of alkaloids, particularly the N-oxides of tertiary alkaloids, are well-known laboratory products, easily prepared from the original base. As early as the 1920s quite extensive pharmacological and toxicological comparisons had been made of common alkaloids such as morphine, strychnine and hyoscyamine and their corresponding N-oxides. Some enthusiasm for the clinical use of N-oxides was engendered by their purported delayed-release properties, low toxicities and low addictive properties compared with the corresponding tertiary alkaloids.
EXTRACTION OF ALKALOIDS
Extraction methods vary with the scale and purpose of the operation, and with the raw material. For many research purposes chromatography gives both speedy and accurate results. However, if an appreciable quantity of alkaloid is required, one of the following general methods will usually serve.
Process B
Large-scale extractions based on the above principles are sometimes done in the field and the crude mixtures of alkaloids afterwards sent to a factory for separation and purification. This has been done for both cinchona and coca alkaloids in South America and Indonesia, the crude alkaloids then being sent to Europe, USA or Japan for purification. The separation and final purification of a mixture of alkaloids may sometimes be done by fractional precipitation or by fractional crystallization of salts such as oxalates, tartrates or picrates. Chromatographic methods are particularly suitable if the mixture is a complex one and if small quantities of alkaloids will suffice. Supercritical fluid extraction (Chapter 17), although not yet applied to many alkaloids, will probably become of increasing importance for these compounds.
Cell, tissue and organ culture
The production of alkaloids using cell, tissue and organ cultures has now been extensively investigated for its commercial potential, as a means of obtaining new alkaloids and for elucidating biosynthetic pathways. These aspects are considered in Chapter 13 and under individual drugs.
FUNCTIONS OF ALKALOIDS IN PLANTS
Cordell GA, editor. The alkaloids. Chemistry and pharmacology, Vols 41–. London: Academic Press, 1991. This is a continuation of the original Manske and Holmes series started in 1950. Vol. 53. A Brossi as editor appeared in followed by, Vols 23–41, 2000.
Roberts MR, Wink M, editors. Alkaloids. Biochemistry, ecology and medical applications. New York: Plenum, 1998.
Southon IW, Buckingham J. Dictionary of alkaloids. London: Chapman and Hall, 1989.
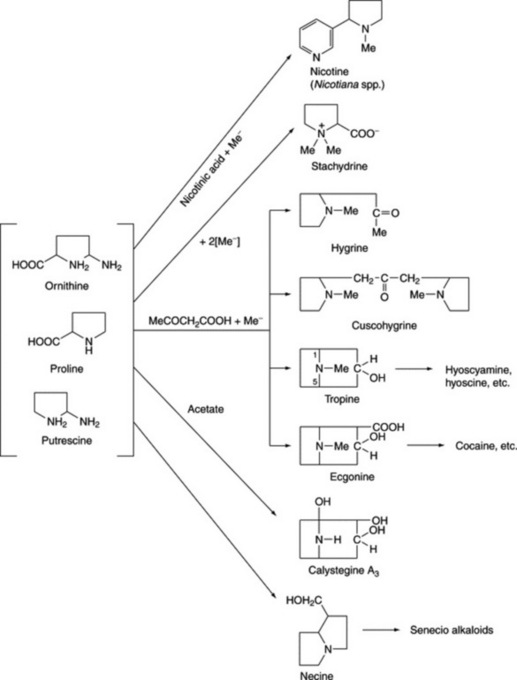
ORNITHINE-DERIVED ALKALOIDS
As indicated in Fig. 26.2, the amino acid ornithine, its decarboxylation product, putrescine, and proline constitute the basic unit of the tropane, ecgonine, nicotine (pyrrolidine ring), necine and stachydrine groups of alkaloids. Biogenetically ornithine is linked to arginine (Fig. 18.13); putrescine can also be formed from arginine without the involvement of ornithine and this has led to problems in the understanding of the stereospecific incorporation, or otherwise, of precursors into particular alkaloids, see below. Pharmaceutically, the tropane group is important.
TROPANE ALKALOIDS
enzymatic hydrolysis but the chemical treatment converts it to the more stable geometric isomer, oscine).
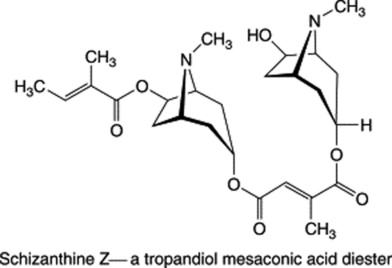
These three specific alkaloids are confined to the Solanaceae, in which some 40 different ester bases of the tropane type have now been discovered; they constitute an interesting chemotaxonomic study within the family. Examples of tropanol esters are given in Table 26.2. Dimeric and trimeric tropanol ester alkaloids involving the dicarboxylic acids mesaconic and itaconic acids are found in Schizanthus. For isolations from S. porrigens see O. Muñoz and S. Cortés, Pharm. Biol., 1998, 36, 162, and from S. hookeri see M. Jordan et al., Phytochemistry, 2006, 67, 570. Other tropane bases occur in the Erythroxylaceae (see cocaine in coca leaves), Convolvulaceae, Dioscoreaceae, Rhizophoraceae, Cruciferae and Euphorbiaceae.
Table 26.2 Examples of ester components of tropane alkaloids of the Solanaceae.
| Genera of pharmaceutical interest | Atropa, Acnistus, Scopolia, Physochlaina, Przewalskia, Hyoscyamus, Physalis, Mandragora, Datura, Solandra, Duboisia, Anthocercis |
| Tropanol components of esters | 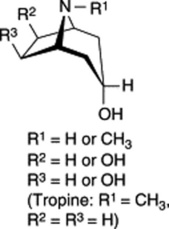 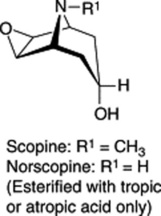  |
| Esterifying acids | Acetic, propionic, isobutyric, isovaleric, 2-methylbutyric, tiglic, nonanoic, tropic, atropic, 2-hydroxy- 3-phenylpropionic, 2,3-dihydroxy-2-phenylpropanoic, p-methoxyphenylacetic, anisic |
BIOGENESIS OF TROPANE ALKALOIDS
Tropane moiety
Early work with isotopes indicated that ornithine and acetate were precursors of the tropane nucleus; later, the incorporation of ornithine was shown to be stereospecific. Hygrine can also serve as a precursor of the tropane ring but is not now considered to lie on the principal pathway. The N-methyl group of the tropane system can be supplied by methionine and can be incorporated at a very early stage of biosynthesis, as demonstrated by the intact incorporation of N-methylornithine into hyoscine and hyoscyamine of Datura metel and D. stramonium. Early involvement of the N-methyl group was reinforced by the isolation in 1981 of naturally occurring δ-N-methylornithine from belladonna plants. Also supporting the stereospecificity of the ornithine incorporation was the work of McGaw and Woolley (Phytochemistry, 1982, 21, 2653) which showed that for D. meteloides the C-2 of hygrine was specifically incorporated into the C-3 of the tropine moiety of the isolated alkaloid. Putrescine (the symmetrical diamine formed by the decarboxylation of ornithine) and its N-methyl derivative also serve as precursors, which, taken in conjunction with the stereospecific incorporation of ornithine, makes it difficult to construct a single pathway for tropane ring formation. A scheme for the biogenesis of the tropane moiety, consistent with the above findings, is shown in Fig. 26.3.
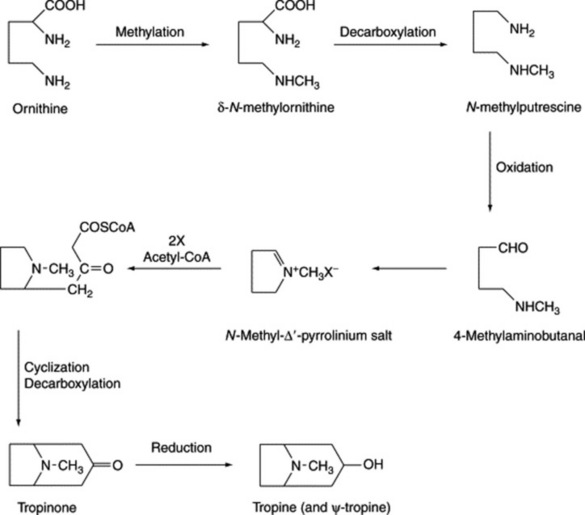
Fig. 26.3 Possible biogenetic routes for tropine and pseudotropine (see text for additional comments).
It will be observed from Fig. 26.3 that the reduction of tropinone yields both tropine (3α-hydroxytropane) and pseudotropine (3β-hydroxytropane). These reductions are brought about by two independent tropinone reductases (EC 1.1.1.236), often referred to as TR-I and TR-II, which accept NADPH as coenzyme. After considerable research involving principally D. stramonium root cultures both enzymes were separately purified and characterized. Furthermore, cDNA clones coding for the two separate enzymes TR-I and TR-II have been isolated and shown to involve polypeptides containing 272 and 260 amino acids respectively. These clones were expressed in Escherichia coli and the same reductive specificity demonstrated as for the natural TRs isolated from plant material.
As indicated in Table 26.2 for solanaceous alkaloids, hydroxyls and ester groups are also common at C-6 and C-7 (R2 and R3) of the tropane ring system. Current evidence suggests that hydroxylation of these carbons probably occurs after the C-3 hydroxyl has been esterified.
Acid moiety
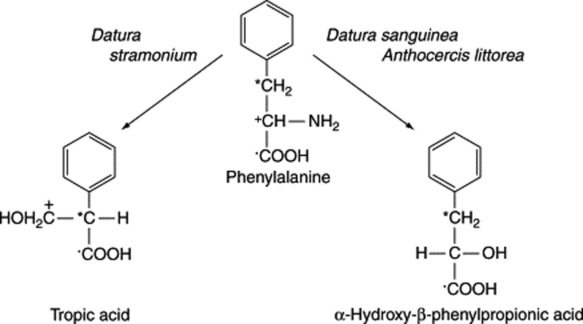
The tropic acid fragment of hyoscine and hyoscyamine is derived from phenylalanine, as is the α-hydroxy-β-phenylpropionic acid (phenyllactic acid) of the tropane alkaloid littorine. The specific incorporations obtained with phenylalanine are given in Fig. 26.4. The sequence involved in the rearrangement of the side-chain in the conversion of phenylalanine to tropic acid has been the subject of longstanding debate. Ansarin and Woolley (Phytochemistry, 1994, 35, 935), by feeding phenyl [1,313C2]lactic acid to D. stramonium and examining the 13C-NMR spectra of the subsequently isolated hyoscine and hyoscyamine, have substantiated the hypothesis that tropic acid is formed by an intramolecular rearrangement of phenyllactate. Furthermore, it has been demonstrated that hyoscyamine is biosynthesized from littorine by a process involving the intramolecular rearrangement of the phenyllactate moiety of the alkaloid. In concordance with this, transformed roots of Datura stramonium will convert exogenously added littorine to hyoscyamine (35% metabolism recorded) but, in contrast, exogenously added hyoscyamine is not metabolized to littorine.
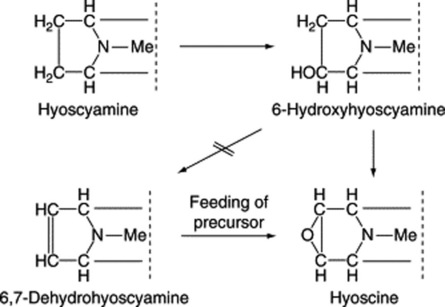
Biogenesis of hyoscine (scopolamine)
Work initiated by Romeike in 1962 showed that hyoscine appeared to be formed in the leaves of D. ferox from hyoscyamine via 6-hydroxyhyoscyamine and 6,7-dehydrohyoscyamine. The former intermediate has been well substantiated, as indicated below, and occurs in quantity in some other genera (Scopolia, Physochlaina, Przewalskia) but the latter, although incorporated into hyoscine when fed as a precursor to D. ferox, has never been isolated from normal plants. Some 25 years later Hashimoto’s group, using Hyoscyamus niger cultured roots, isolated and partially purified the enzyme responsible for the conversion of hyoscyamine to 6-hydroxyhyoscyamine. They used this enzyme to prepare [6-18O]-hydroxyhyoscyamine from hyoscyamine and showed that when the labelled compound, fed to Duboisia myoporoides, was converted to hyoscine the 18O was retained, thus eliminating 6,7-dehydrohyoscyamine from the pathway (Fig. 26.5). For this reaction to proceed the epoxidase enzyme requires 2-oxo-glutarate, ferrous ions and ascorbate as cofactors, together with molecular oxygen.
Griffin WJ, Lin GD. Chemotaxonomy and geographical distribution of tropane alkaloids. Phytochemistry. 2000;53:623-637.
Lounasmaa M, Tamminen T. Cordell GA, editor. The alkaloids. Chemistry and pharmacology, Vol 44. London: Academic Press, 1993.
A review with 484 references listing all known tropane alkaloids
Robins RJ, Walton NJ. The biosynthesis of tropane alkaloids. The alkaloids. Chemistry and pharmacology, 1993.
STRAMONIUM LEAF
Plant
D. Stramonium is a bushy annual attaining a height of about 1.5 m and having a whitish root and numerous rootlets. The erect aerial stem shows dichasial branching with leaf adnation. The stem and branches are round, smooth and green. The flowers are solitary, axillary and short-stalked. They have a sweet scent. Each has a tubular, five-toothed calyx about 4.5 cm long, a white, funnel-shaped corolla about 8 cm long, five stamens and a bicarpellary ovary. The plant flowers in the summer and early autumn. The fruit is originally bilocular but as it matures a false septum arises, except near the apex, so that the mature fruit is almost completely four-celled. The ripe fruit is a thorny capsule about 3–4 cm long. Stramonium seeds (see Fig. 41.6) are dark brown or blackish in colour, reniform in outline and about 3 mm long. The testa is reticulated and finely pitted. A coiled embryo is embedded in an oily endosperm.
Microscopical characters
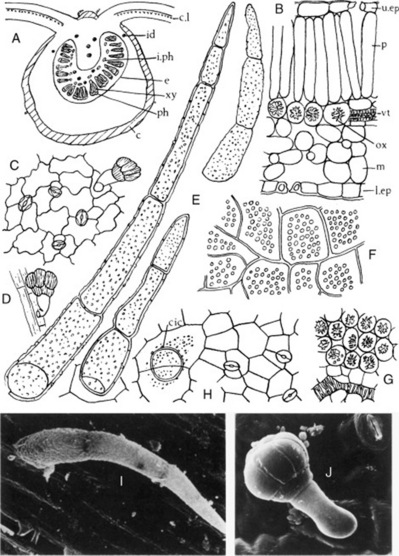
A transverse section of a leaf (Fig. 26.6) shows that it has a bifacial structure. Both surfaces are covered with a smooth cuticle and possess both stomata and hairs. Cluster crystals of calcium oxalate are abundant in the mesophyll (Fig. 26.6F, G), and microsphenoidal and prismatic crystals are also found. The stomata are of the anisocytic and anomocytic types. The epidermal cells have wavy walls, particularly those of the lower epidermis. The uniseriate clothing hairs are three- to five-celled, slightly curved, and have thin, warty walls (Fig. 26.6E). The basal cell is usually more than 50 μm long (distinction from D. metel). Small glandular hairs with a one- or two-celled pedicel and others with a two-celled pedicel and an oval head of two to seven cells are also found. If portions of the leaf are cleared with chloral hydrate solution, the abundance of the cluster crystals of calcium oxalate and their distribution with regard to the veins may be noted.
Constituents
Stramonium usually contains 0.2–0.45% of alkaloids, the chief of which are hyoscyamine and hyoscine, but a little atropine may be formed from the hyoscyamine by racemization. At the time of collection these alkaloids are usually present in the proportion of about two parts of hyoscyamine to one part of hyoscine, but in young plants hyoscine is the predominant alkaloid. The TLC test for identity given in the BP/EP enables other Datura species containing different proportions of alkaloids to be detected. The larger stems contain little alkaloid and the official drug should contain not more than 3% stem with a diameter exceeding 5 mm. Stramonium seeds contain about 0.2% of mydriatic alkaloids and about 15–30% of fixed oil. The roots contain, in addition to hyoscine and hyoscyamine, ditigloyl esters of 3,6-dihydroxytropane and 3,6,7-trihydroxytropane, respectively, and a higher proportion of alkamines than the aerial portions. For a recent report on the distribution of alkaloids in different organs and in three varieties of D. stramonium, see S. Berkov et al., Fitoterapia, 2006, 77, 179. D. stramonium cell and root cultures have been considerably utilized in biogenetic studies.
Prepared Stramonium BP/EP is the finely powdered drug adjusted to an alkaloid content of 0.23–0.27%.
HYOSCYAMUS LEAF
Plant
Henbane is a biennial (var. α-biennis) or annual (var. β-annua) plant. It is found wild, chiefly near old buildings, both in the UK and in the rest of Europe, and is widely cultivated. Before examining commercial henbane leaves it is advisable to study growing plants or herbarium specimens. The differences tabulated in Table 26.3 should be noted.
Table 26.3 Comparison of commercial varieties of hyoscyamus.
| First-year biennial | Second-year biennial | Annual |
|---|---|---|
| Stem very short | Stem branched and up to 1.5 m high | Stem simple and about 0.5 m high |
| Leaves in a rosette near the ground. Ovate-lanceolate and petiolate, up to 30 cm long, the lamina being up to 25 cm long. Hairy | Leaves sessile, ovate-oblong to triangular-ovate. 10–20 cm long. Margin deeply dentate or pinnatifid. Very hairy, especially in the neighbourhood of the midrib and veins | Leaves sessile. Smaller than those of the biennial plant, with a less incised margin and fewer hairs |
| Does not normally flower in the first year | Flowers May or June. Corolla yellowish with deep purple veins | Flowers July or August. Corolla paler in colour and less deeply veined |
Henbane flowers have the formula K(5), C(5), A5, G(2). The hairy, five-lobed calyx is persistent. The fruit is a small, two-celled pyxis (see Fig. 41.6B), which contains numerous seeds.
History
Henbane, probably the Continental H. albus, was known to Dioskurides and was used by the ancients. Henbane was used inEngland during the Middle Ages. After a period of disuse in the eighteenth century the drug was restored to the London Pharmacopoeia of 1809, largely owing to the work of Störck.
Microscopical characters
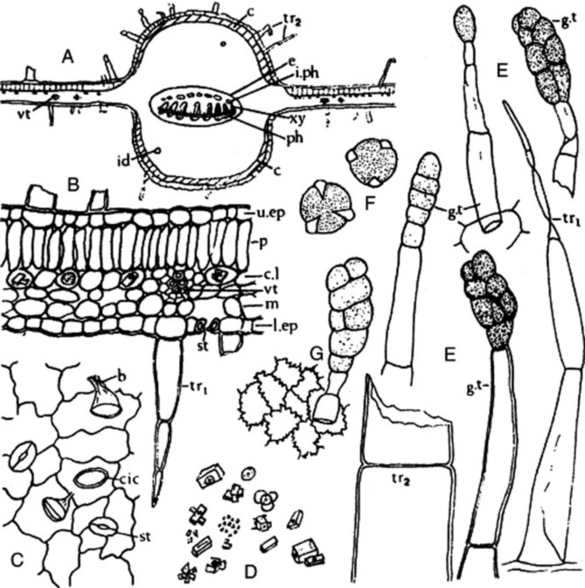
A transverse section of a henbane leaf shows a bifacial structure (Fig. 26.7A). Both surfaces have a smooth cuticle, epidermal cells with wavy walls, stomata of both anisocytic and anomocytic types, and a large number of hairs, which are particularly abundant on the midrib and veins. The hairs are up to 500 μm long; some are uniseriate and two to six cells long, while others have a uniseriate stalk and a large, ovoid, glandular head, the cuticle of which is often raised by the secretion (Fig. 26.7E). Similar hairs are found on the stems. The spongy mesophyll contains calcium oxalate, mainly in the form of single and twin prisms, but clusters and microsphenoidal crystals are also present (Fig. 26.7B,D). The broad midrib contains a vascular bundle, distinctly broader than that of stramonium, showing the usual bicollateral arrangement, which is also to be seen in the stems. The mesophyll of the midrib is made up of two thin zones of collenchyma immediately within the epidermi and a ground mass of colourless parenchyma showing large, intercellular air spaces and containing prisms or, occasionally, microsphenoidal crystals of calcium oxalate.
The calyx possesses trichomes and stomata, as in the leaf. The corolla is glabrous on the inner surface but exhibits trichomes on the outer surface, particularly over the veins (Fig. 26.7G). Those cells of the corolla which contain bluish anthocyanins turn red with chloral hydrate solution. Numerous pollen grains are present, about 50 μm diameter, tricolpate with three wide pores and an irregularly, finely pitted exine (Fig. 26.7F). The testa of the seeds has an epidermis with lignified and wavy anticlinal walls, and sclereids are present in the pericarp.
Constituents
Henbane leaves contain about 0.045–0.14% of alkaloids and yield about 8–12% of acid-insoluble ash (BP/EP 2001 not more than 12%). Hyoscyamine and hyoscine are the principal alkaloids. The petiole appears to contain more alkaloid than the lamina or stem.
Allied drugs
Hyoscyamus aureus and H. pusillus are two species which produced hyoscine as the principal alkaloid.
BELLADONNA LEAF
A. belladonna is cultivated in Europe and the USA.
Plant
The flowers appear about the beginning of June. They are solitary, shortly stalked, drooping and about 2.5 cm long. The corolla is campanulate, five-lobed and of a dull purplish colour. The five-lobed calyx is persistent, remaining attached to the purplish-black berry. The latter is bilocular, contains numerous seeds and is about the size of a cherry (see Fig. 41.6C). In the USA the plant is often known as the ‘Poison Black Cherry’, while the German name is ‘Tollkirschen’ (i.e. Mad Cherry). A yellow variety of the plant lacks the anthocyanin pigmentation; the leaves and stems are a yellowish-green and the flowers and berries yellow.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree