(1)
Canberra, ACT, Australia
Summary
Apical periodontitis is an inflammatory disorder of peri-radicular tissues caused by aetiological agents of endodontic origin. The pulp–dentine complex has only a limited ability to withstand microbiological, mechanical, chemical or thermal insults by means of reactionary or reparative dentine. Polymicrobial bacteria will eventually colonise the pulp resulting in a standoff between host defence mechanisms and bacterial colonisation. Eventually bacteria will reach the apical tissues resulting in clinical and radiographic signs and symptoms associated with apical periodontitis. Effective treatment strategies should be aimed at reducing this intra-canal biofilm to levels below a critical threshold conducive to healing.
Clinical Relevance
The primary aetiological factor for persisting apical periodontitis is intra-canal bacteria or bacterial biofilms. Treatment options for the disease consist of chemo-mechanical disruption of the biofilm removing or at least reducing the intra-canal microbial load to subcritical numbers achieved by root canal instrumentation, use of sodium hypochlorite irrigation, intra-canal antibacterial medication and prevention of re-infection by obturation. Any deviation from such protocols can only lead to persistent microbes that may or may not pose future hindrance for healing.
2.1 Overview of the Pulp–Dentine Complex
The dental pulp is a specialised connective tissue entirely enclosed by dentine consisting of the pulp periphery and pulp proper. The peripheral pulp can be distinguished into three further zones including the pseudo stratified layer of the highly differentiated dentine producing odontoblast cells, a sub-odontoblastic 40-μm cell-free zone and a cell-rich zone. The terminal branches of the sensory and autonomic nerve fibres are located in the sub-odontoblastic zone. The central pulp core consists of mainly fibroblasts, collagen and elastin fibres, large blood vessels and nerve bundles. The entire pulp is embedded in a gel-like ground substance [1].
The odontoblasts are responsible for production of mineralised dentine. Dentine is permeated by millions of tubules each containing a cellular process from an odontoblast. In a fully developed tooth, the dentinal tubules have a diameter of 3 μm at the outer periphery of dentine with a density of approximately 15,000/mm2. As the dentinal tubules converge towards the pulp, they are more closely packed together with diameters reducing to 1 μm with a density of 65,000/mm2 [1, 2].
The pulp–dentine complex has the ability to respond to microbiological, mechanical, thermal or chemical stimuli and insults, which are responsible for inflammation within the pulp. Mild insults may result in increased dentinogenesis as a means of a protective mechanism whereby increased peri-tubular dentine formation responsible for the formation of sclerotic dentine can occur. Tertiary dentine, either reactionary or reparative in origin, can also be formed in response to dentine injuries or toxic products that reach the pulp–dentine complex. Reactionary dentine, typically produced by pre-existing odontoblasts, may be a response to a freshly cut cavity or a response to the restorative interface. Newly differentiated odontoblastoid cells, on the other hand, form reparative dentine, when the primary odontoblast is irreversibly damaged. Growth factors such as transforming growth factor-β are responsible for the initiation of odontoblast differentiation and stimulation of dentine formation. Release of growth factors typically occurs during carious attacks to the tooth and injuries sustained following cavity preparation and subsequent restoration of the tooth [2–4].
2.2 Role of Bacteria in Peri-apical Disease
The presence of organisms in a necrotic dental pulp was observed by Miller [5], but their role in the development of apical periodontitis was not confirmed until 70 years later. A definitive relationship between bacteria and peri-apical disease is credited to Kakehashi and colleagues, demonstrating that a normal oral microbiota was necessary to produce pathologic changes resulting from untreated experimental pulp exposures [6]. Numerous human and animal studies have confirmed the importance of bacterial infection in the development of apical periodontitis [7–11]. The polymicrobial nature of the root canal flora has been studied extensively, and obligate anaerobes, mainly Gram-negative bacteria (Prevotella spp., Porphyromonas spp., Fusobacterium spp., Veillonella spp.) and some Gram-positive bacteria (Actinomyces spp., Propionibacterium spp., Peptostreptococcus spp., Eubacterium spp.) have been suggested as putative pathogens [9, 10, 12–14]. Using a monkey model to evaluate the peri-apical response to indigenous bacterial infections, it was shown that, over time, facultative anaerobic species and obligate anaerobes in combination have been found to be capable of inducing inflammation. It was found that bacteria inoculated in combination in root canals had the capacity to induce peri-apical changes and in such cases the bacteria were re-isolated in equal proportions to those recovered in the primary infection [15, 16].
Uncertainty about the bacterial origin of peri-apical disease had allowed other aetiological theories to become established. These included the disproved hollow tube theory stating that necrotic pulp tissue and stagnant tissue fluid were responsible for irritation of the peri-apical tissues [16–18]. Necrotic pulp tissue [19], bacterial products [20] and viruses [21] have all been investigated, but no definitive causal evidence was found.
2.3 Route of Bacterial Entry into the Root Canal System
The root canal represents a special environment in which selective pressures result in the establishment of a restricted group of the oral flora [22, 23]. This selection process begins by a mode of entry, which may ultimately affect the pattern of infection within the root canal. The major pathways of pulpal contamination are open communication to the salivary plaque microorganisms, exposed dentinal tubules [24–27], carious exposure through crown or root [28], lateral canals [29], microleakage [25–27, 30, 31], traumatic injury and anachoresis [32–34]. This latter route, whereby bacteria reach the pulp and canal system via severed blood vessels in the periodontium, was proposed to explain findings in which bacteria were isolated from teeth with necrotic pulps and apparently ‘intact crowns’ [9, 10, 12]. Results of experiments carried out by Möller and Delivanis and Fan [11, 35] conclusively exclude this as a potential cause. Microleakage through enamel cracks has been identified as the most probable route of endodontic infection [24]. Pulpal infection is also possible through exposed dentinal tubules at the cervical root surface because of congenitally missing cementum at the cement–enamel junction, which occurs in 10 % of the population; exposed dentine in periodontitis; or after loss of cemental covering due to periodontal treatment [36].
2.4 The Spatial Distribution of Microflora Within the Root Canal System
A light microscopy (LM) study described the distribution of microorganisms within the root canal as being polar in nature with a greater concentration of bacteria found in the bucco-lingual plane. It was demonstrated that bacteria were able to penetrate up to half the thickness of dentine with more bacteria present in the coronal segment as compared to the apical [37]. With LM, by using specific stains, i.e. Gram stain, it is possible to visualise and characterise cells by their colouration (see Fig. 2.1). However, by virtue of using such stains, several bacteria may display similar staining characteristics making identification difficult.
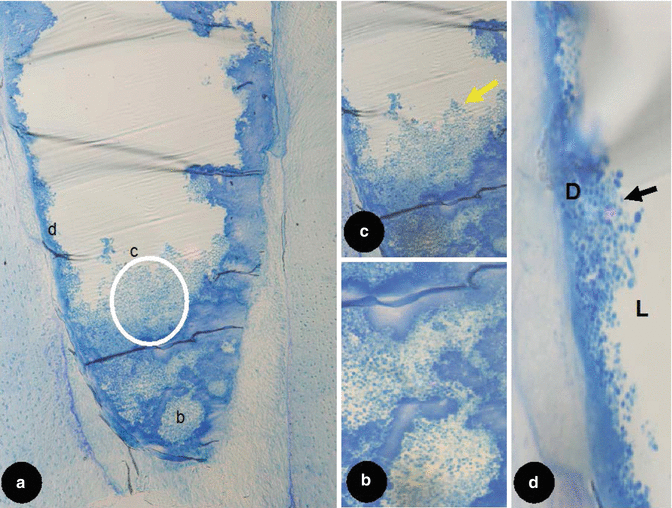

Fig. 2.1
Light microscopy longitudinal section of an artificially created endodontic infection within a tooth created using human saliva, serum and a constant depth film fermentor after 28 days. Original magnifications: (a) 10×, (b) 50×, (c) and (d) 1,000×. Note: the presence of biofilm (yellow arrowhead) and dense aggregate of bacteria (black arrowhead) sticking to the dentinal wall (D) along with a loose collection of bacteria suspended within the lumen (L). (white circle) this area is magnified in (c) and (d)
A significantly greater percentage of coccoids and rods have been seen in the coronal third than in the apical third of root canals of teeth with pulpal necrosis in a dark-field study. The percentage of filaments and spirochaetes, although not significant, was shown to be slightly higher in the apical than in the coronal root segment. The quantification method used was based on the observation of 200 bacteria, which were classified into morphological categories (coccoids, straight rods, filaments, spirochaetes and motile rods) [38].
The morphological structure of the root canal flora as seen by both LM and transmission electron microscopy (TEM) (see Figs. 2.2 and 2.3) has been described as consisting of cocci, rods, filamentous organisms and spirochaetes. Structurally, the bulk of the root canal flora was described as loose collections of a variety of morphologically distinct planktonic bacteria suspended in an apparently moist canal lumen. Less frequently, the bacterial flora formed clusters of ‘self-aggregating’ colonies of one distinct type or ‘coaggregating’ communities of several types sticking to the dentinal wall of the root canal or existing free among vast numbers of PMNs in the canal lumen [39]. Further work demonstrated and confirmed the great anatomical complexity of the root canal and the ecological organisation of the flora into protected sessile biofilms [40].
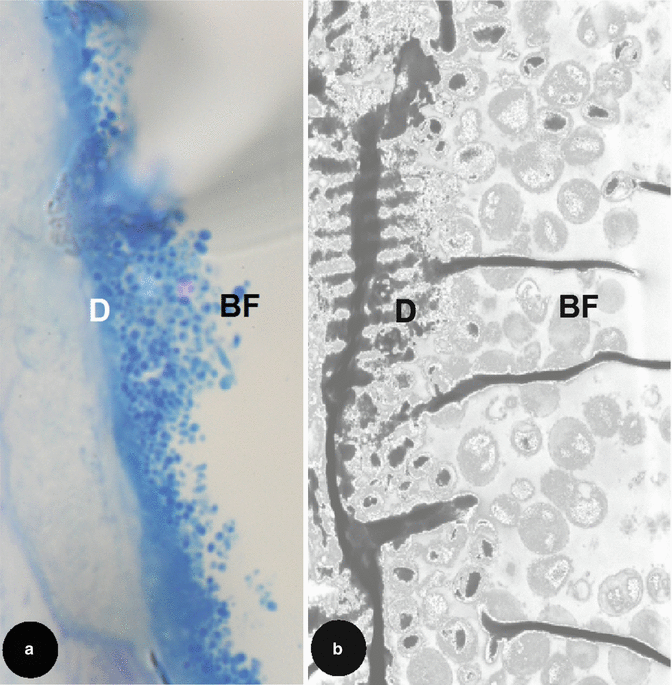
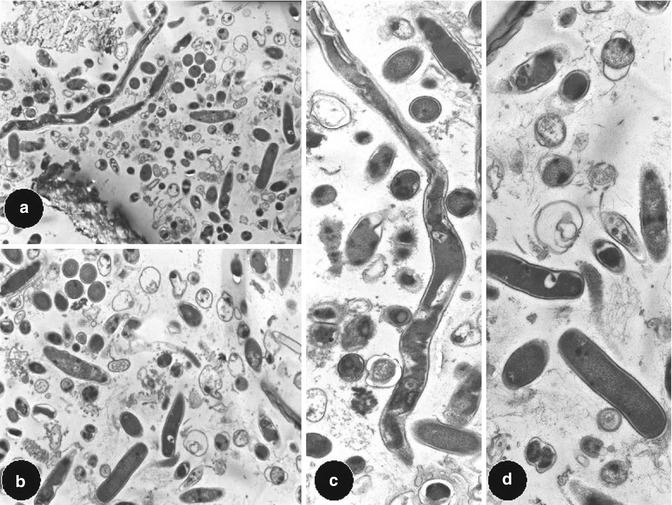

Fig. 2.2
Light microscopy longitudinal section of an artificially created endodontic infection within a tooth created using human saliva, serum and a constant depth film fermentor after 28 days. Original magnifications: (a) 1,000× and transmission electron microscopy view (b) 4,000×. Note: structurally at the dentine (D) and biofilm (BF) interface, a variety of morphologically distinct but taxonomically unidentifiable bacteria can be seen

Fig. 2.3
Transmission electron microscopy views of longitudinal section of an artificially created endodontic infection within a tooth created using human saliva, serum and a constant depth film fermentor after 28 days. Original magnifications: (a) and (b) 1,000× and (c) and (d) 4,000×. Note: morphological appearance of cocci, rods and filaments associated with a polymicrobial infection within a tooth
Further SEM studies reported the existence of cocci, which seemed to attach themselves to filaments giving a ‘corncob’-like appearance in the apical 2 mm of root canals associated with peri-apical disease [41] (see Fig. 2.4). A study using SEM examining the entire length of the canal showed a continuous biofilm along the canal wall [42].


Fig. 2.4
Scanning electron microscopy views of longitudinal section of an artificially created endodontic infection within a tooth created using human saliva, serum and a constant depth film fermentor after 28 days. Original magnification 1,000×. Note: morphological appearance of coccoid and filamentous organism
2.5 Dentinal Tubule Invasion
Some research has been done on the penetration of bacteria into the dentinal tubules. Bacteria were seen to invade the dentinal tubules in early studies, and the extent of bacterial invasion was seen to be time dependent [37]. Later studies investigated the depth of penetration of the dentinal tubules, and the reported depths vary from 150 to 2,000 μm, consisting of predominantly Gram-positive rods and cocci [14, 42, 43].
Bacterial penetration of teeth with peri-apical radiolucencies was examined using culturing and histological techniques. Bacteria were found in 62 % of teeth in the layer closest to the cementum layer. Microbiological examination of dentine samples taken at different distances from the canal lumen revealed 90 % of the dentine grindings to show quantitative evidence for penetration of bacteria towards the CEJ. Gram staining of histological specimens, however, could only detect bacteria from the pulpal–dentinal junction as far as 375 μm. Perhaps this discrepancy was associated with contamination from adjacent layers of dentine during processing or attributed to the lack of sensitivity of LM to detect the low numbers of bacteria from the small dentine sample [14]. Intact cementum may be a limiting factor in bacterial penetration from the pulpal surface, and its absence enhances bacterial penetration [24, 44].
It has been speculated that bacterial adhesion specificity plays a major role in determining the invasion of the dentinal tubules. Recognition of type І collagen may facilitate bacterial adhesion to dentine [45]. Antigen І/ІІ family polypeptides produced by some oral streptococci mediate primary binding of bacteria to intratubular collagen type І. Once the cells had adhered to the collagen, colonisation down the length of the tubule occurred following an upregulation of antigen І/ІІ polypeptides, stimulated by the release of peptides during the demineralisation process. This results in an increase in community growth within and along the dentinal tubule. It has been hypothesised that tissue fluid from the periodontal ligament and alveolar bone bathing the root of a tooth may provide sufficient nutrition to bacteria within radicular dentinal tubules or the obturated root canals [46], accounting for the presence of streptococci and enterococci in failed endodontically treated cases [47, 48].
2.6 Fate of Bacteria Within the Root Canal System and Their Interactions
The microbial composition of an infected root canal is determined by [1] the route by which the bacteria gain access to the root canal and [3] the number and quality of ecological factors [49]. In autogenously infected monkey teeth, the most common bacterial strains were found to be facultative anaerobic streptococci, obligate anaerobic non-sporulating bacteria and coliform rods [11]. Further studies on monkeys found that when combinations of bacterial species were retrieved from infected root canals and inoculated into uninfected root canals in equal amounts, the original proportions became re-established, indicating that selective mechanisms allow certain bacteria to survive and multiply more than others [50].
In a succession of studies, the associations between microbial species in dental root canal infections of teeth with intact pulp chamber walls were examined, and it was concluded that commensal and antagonistic relationships existed. Strong positive associations were found between Fusobacterium nucleatum and Peptostreptococcus micros, Porphyromonas endodontalis, Selenomonas sputigena and Wolinella recta. There was also a positive association between Prevotella intermedia and P. micros, P. anaerobius and the eubacteria. Species of streptococci, Propionibacterium propionicus, Capnocytophaga ochracea and Veillonella parvula showed no or negative associations with the other bacteria [22, 23].
Certain bacteria only become pathogenic in the presence of other species. It has been suggested that bacteriocins (proteins which have the capacity to inhibit growth of a limited number of species) are responsible for negative associations [51].
2.7 Provision of Nutrients
The root canal is a unique environment providing a sanctuary to a biologically select anaerobic milieu, which interacts with microbial factors and the availability of nutrients. This restricted interacting group of species are dependent on one another for nutrition as the metabolism of one species supplies essential nutrients for growth of other members of the populations [22]. Nutrients may be derived from the oral cavity (saliva), degenerating connective tissue, dentinal tubule contents or a serum-like fluid from the peri-apical tissues [52, 53]. Such predominating factors, within the root canal environment, permit the growth of anaerobic bacteria fermenting amino acids and peptides apically [22, 23], whereas bacteria that previously obtained energy by fermenting carbohydrates may be restricted more coronally due to dominance of such nutrients there. Growth of mixed bacterial populations may depend on a food chain in which metabolites of one species supply essential nutrients for the growth of other members of the population [23] (Fig. 2.5).
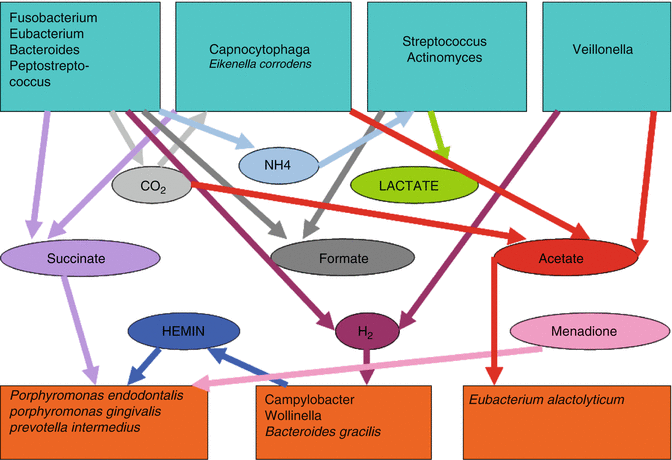

Fig. 2.5
Possible nutritional relationships between bacteria in an infected root canal (Adapted from Sundqvist [22])
The relatively increased proportion of strict obligate anaerobic organisms and the reduction in facultative anaerobic organisms, over time, in the apical portion are not only nutrient driven but also partly due to the decrease in available oxygen [15, 50]. It is likely that within a tooth with necrotic pulp tissue, apical infection is maintained mainly by apical seepage of inflammatory/serum exudate or possibly blood (such as traumatic instrumentation) [54].
It has been suggested that bacteria invading dentinal tubules may also gain nourishment from interstitial fluid originating from alveolar bone and periodontal ligament and as such would resemble serum. This fluid may sustain cells within the tubules allowing for subsequent growth [46].
2.8 Pathogenesis of Peri-apical Disease
Peri-apical disease is the result of the interactions between bacteria (and their by-products) and the host defences. The non-specific and specific branches of the host defences are recruited to defend against the potential invasion of the body by bacteria. The peri-apical lesion represents resorption of bone away from the source of infection, whereby space is created for the migration of the body’s defensive elements to counteract the ongoing infection. In the early stages, following acute inflammation, as a result of host/bacterial interactions at the peri-apex of an infected tooth, slight widening of the periodontal ligament space may be evident radiographically. In the absence of treatment intervention, bone destruction progresses from an acute to chronic state resulting in marked radiographic radiolucency that is clinically detectable. In animal models, the area of bone destruction has been shown to increase with time with a rapid phase between 7 and 15 days and stabilisation after 30 days. The proportion of anaerobic bacteria, species present and interaction with host dictates the amount of bone destruction. Clinically there can be a wide range in presentation from the absence of any signs and symptoms (chronic apical periodontitis), a discharging sinus (chronic suppurative apical periodontitis) and the presence of pain and swelling (acute exacerbation of chronic apical periodontitis) [55–57].
2.9 Biofilms and Endodontics
A biofilm is defined as an aggregation of bacteria associated with a solid surface, embedded in an extracellular matrix of polysaccharide and other metabolic products [58]. Plaque is a classic example, which becomes spatially differentiated after attachment and growth of pioneer species, allowing secondary colonisers, not able to colonise the surface alone, to adhere.
As the biofilm develops, gradients in factors such as oxygen levels, redox potentials and pH develop and permit the co-existence of species that would be incompatible with each other in a homogenous environment. Microbial communities usually develop on environmentally exposed surfaces as a biofilm. Bacteria, which first colonise a habitat, have been termed ‘pioneer species’. The metabolism of these organisms can modify the habitat and local environment to such an extent as to make conditions more suitable for the growth of ‘secondary colonisers’ with more demanding requirements [59].
The early colonisers of tooth enamel include streptococci (especially Streptococcus sanguis and Streptococcus oralis) (Nyvar 1987) and Neisseria spp. that consume oxygen and liberate carbon dioxide and hydrogen, creating micro-environments suitable for facultative anaerobes and eventually obligate anaerobes [60].
The process of changes in bacterial species and their proportions in a habitat during colonisation is termed ‘succession’. Eventually, the composition of the microbial community at a site becomes stable over time, known as the ‘climax community’. The composition of this climax community can, however, change further depending on key environmental factors such as nutritional status or local environmental conditions at a site, which ultimately control the composition or activity of microbial communities during development [59].
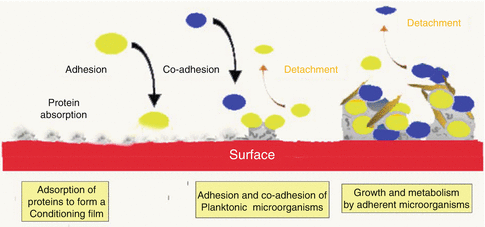
Free-floating bacteria existing in an aqueous environment, so-called planktonic microorganisms, are a prerequisite for biofilm formation. Planktonic organisms in saliva serve as the primary source for the organisation of a specific biofilm. The establishment of a micro-community on a surface seems to follow essentially the same series of developmental stages, including deposition of a conditioning film, adhesion and colonisation of planktonic organisms in a polymeric matrix, co-adhesion of other organisms and detachment of biofilm microorganisms into the surroundings [61] (Fig. 2.6).
As far as endodontic infections are concerned, the ‘biofilm concept’ has thus far gained limited attention. Bacterial ‘condensations’, representing biofilms, have been discussed either within the framework of appearances on root tips with non-vital pulps [62–64] or on the walls of infected root canals [39–41].
Biofilm structures may provide protection and may allow better resistance to adverse external influences for the organisms incorporated as compared with the planktonic state [65]. From this aspect, the formation of biofilms carries particular clinical significance not only from a host defence mechanism point of view, but also therapeutic efforts including chemical, mechanical and antimicrobial treatment measures. Investigative tools for studying biofilms and the dynamics of ecology in infected root canals should be developed further to enhance our current knowledge, understanding and management of apical periodontitis [66].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree