15 Adverse effects of drugs on the liver
Epidemiology
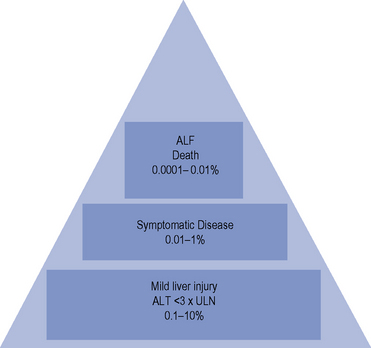
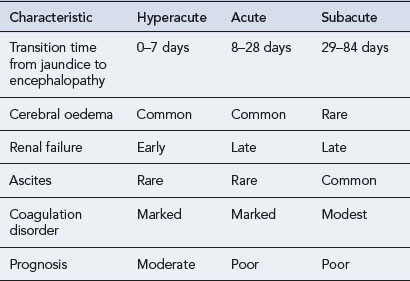
The incidence of drug-induced liver disease (DILD) has continued to rise steadily since the late 1960s, although the incidence of idiosyncratic reactions for most drugs remains low, occurring at therapeutic doses from 1 in every 1000 patients to 1 in every 100,000 patients. DILD is not usually life-threatening; however, for the small number of patients who develop drug-induced acute liver failure (ALF) the prognosis is poor, with a 60–80% mortality rate, unless they receive a liver transplant. The incidence and severity of DILD is shown in Fig. 15.1. It is estimated that 15–40% of ALF cases may be attributable to drugs. Classification of ALF suggests three classes: hyperacute, acute and subacute (Table 15.1).
Table 15.1 Characteristics of the different types of acute liver failure (Richardson and O’Grady, 2002)
Many drugs cause elevated liver enzymes with apparently no clinically significant adverse effect, although in a few patients there may be significant hepatotoxicity. For example, isoniazidcauses elevated liver enzymes in 10–36% of patients taking the drug as a single agent. However, only 1% suffer significant hepatotoxicity, with the liver function tests (LFTs) of the majority returning to normal if therapy is discontinued. Other examples of drugs that elevate liver enzymes are shown in Table 15.2.
Table 15.2 Examples of drugs that elevate liver enzymes
| Drug | Percentage of patients with increase in transaminases |
|---|---|
| Cefaclor | 11% |
| Cefixime | 0.7% |
| Ciprofloxacin | 5% |
| Chlorpromazine | 50% |
| Diclofenac | 15% |
| Donepezil | MHRA reportsa |
| Efavirenz | 4% |
| Heparin/LWMH | 5% |
| Isoniazid | 10–36% |
| Naproxen | 4% |
| Norfloxacin | 0.1% |
| Nevirapine | 12% |
| Niacin | 50% |
| Rifampicin | 15–30% |
| Sodium valproate | 11% |
| SSRIs | MHRA reportsa |
| Statins | 1–2% |
| Sulphonamides | 10% |
a Available at: http://www.mhra.gov.uk
Risk factors
Concurrent diseases and pregnancy
Pre-existing renal disease, diabetes, pregnancy and poor nutrition may all affect the ability of the liver to metabolise drugs effectively and may put the patient at risk of developing liver damage. Table 15.3 summarises the host factors that may predispose a patient to drug hepatotoxicity.
Table 15.3 Examples of host factors that predispose to drug hepatotoxicity
| Host factor | Drug example |
|---|---|
| Pre-existing liver disease | Methotrexate, aspirin, sodium valproate |
| Age | |
| Older | Halothane, isoniazid, chlorpromazine, co-amoxiclav, nitrofuratoin |
| Younger | Aspirin, sodium valproate |
| Gender | |
| Female | Halothane, isoniazid, nitrofurantoin, flucloxacillin, chlorpromazine, erythromycin |
| Male | Sodium valproate (in prepubescent boys), co-amoxiclav |
| Genetics | Halothane, chlorpromazine, phenytoin, carbamazepine, phenobarbital, paracetamol, flucloxacillin |
| Enzyme induction | Paracetamol, halothane, isoniazid, sodium valproate |
| Polypharmacy | NSAIDs if used with other hepatotoxic drugs |
| Isoniazid with rifampicin or pyrazinamide | |
| Sodium valproate with phenytoin | |
| Paracetamol with zidovudine | |
| Concurrent diseases | |
| Diabetes mellitus | Methotrexate |
| Renal failure | Allopurinol, i.v. tetracycline |
| Malnutrition | Paracetamol |
| HIV positive with hepatitis C or B co-infection | Antiretroviral agents |
Aetiology
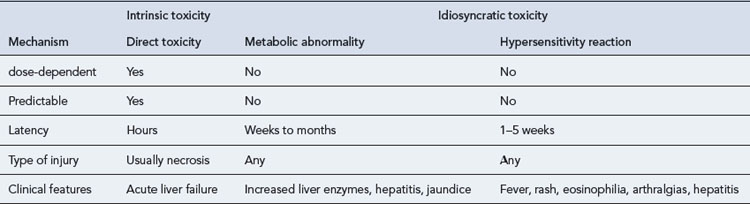
Drug-induced hepatotoxicity may present as an acute insult that may or may not progress to chronic disease, or it can present as an insidious development of chronic disease. The type of lesion may be cytotoxic (cellular destruction) or cholestatic (impaired bile flow). Cytotoxic damage may be further classified as necrotic (cell death) or steatic (fatty degeneration). The liver damage resulting from drug toxicity often presents as a mixed picture of cytotoxic and cholestatic injury. The mechanisms of drug-induced hepatic damage can be divided into intrinsic (type A) and idiosyncratic (type B) hepatotoxicity (Table 15.4). Intrinsic hepatotoxicity is predictable, dose-dependent and usually has a short latency period ranging from hours to weeks. The majority of individuals who take a toxic dose are affected and exhibit the same type of injury. Examples are paracetamol, salicylates, methotrexate and tetracycline. Other examples are presented in Table 15.5. Toxicity may be avoided by ensuring the doses listed are not exceeded.
Table 15.5 Examples of dose-related drug-induced hepatotoxicity
| Drug | Toxic dose |
|---|---|
| Paracetamol | Single dose >10 g |
| Tetracycline | >2 g daily (oral), increased risk of toxicity in pregnancy and renal failure |
| Methotrexate | Weekly dose >15 mg |
| Cumulative dose >2 g in 3 years, increased risk of toxicity in pre-existing liver disease, alcohol abuse, diabetes | |
| 6-Mercaptopurine | >2.5 mg/kg |
| Vitamin A | Chronic use of 40,000 units daily |
| Cyclophosphamide | Daily dose >400 mg/m2 |
| Salicylates | Chronic use >2 g daily |
| Anabolic steroids | High dose >1 month |
| Oral contraceptive | Increased risk with higher oestrogen content, older preparations |
| Duration of treatment | |
| Iron | Single dose >1 g |
Pathophysiology
The range of DILDs is illustrated in Table 15.6. Increased serum level of hepatobiliary enzymes without clinical liver disease occurs with variable frequency between drugs but for some agents it may occur in up to half the patients who receive a drug. This may reflect subclinical liver injury.
Table 15.6 Examples of adverse drug reactions on the liver
| Adverse reaction | Drugs associated with reaction |
|---|---|
| Hepatocellular necrosis | Paracetamol |
| Propylthiouracil | |
| Salicylates | |
| Iron salts | |
| Allopurinol | |
| Dantrolene | |
| Halothane | |
| Ketoconazole | |
| Isoniazid | |
| Mithramycin | |
| Cocaine | |
| ‘Ecstasy’ (methylenedioxymetamphetamine, MDMA) | |
| Fatty liver | Amiodarone |
| Tetracyclines | |
| Steroids | |
| Sodium valproate | |
| l-Asparaginase | |
| Cholestasis | Oral contraceptives |
| Carbimazole | |
| Anabolic steroids | |
| Ciclosporin | |
| Cholestasis with hepatitis | Chlorpromazine |
| Tricyclic antidepressants | |
| Erythromycin | |
| Flucloxacillin | |
| Co-amoxiclav | |
| ACE inhibitors | |
| Sulphonamides | |
| Sulphonylureas | |
| Phenytoin | |
| NSAIDs | |
| Cimetidine | |
| Ranitidine | |
| Trazodone | |
| Granulomatous hepatitis | Phenytoin |
| Allopurinol | |
| Carbamazepine | |
| Clofibrate | |
| Hydralazine | |
| Sulphonamides | |
| Sulphonylureas | |
| Acute hepatitis | Dantrolene |
| Isoniazid | |
| Phenytoin | |
| Chronic active hepatitis | Methyldopa |
| Nitrofurantoin | |
| Isoniazid | |
| Fibrosis and cirrhosis | Methotrexate |
| Methyldopa | |
| Vitamin A (dose-related) | |
| Vascular disorders | Azathioprine |
| Dactinomycin | |
| Dacarbazine |