Chapter 23 Adrenergic mechanisms and drugs
Adrenergic mechanisms
The discovery in 1895 of the hypertensive effect of adrenaline/epinephrine was initiated by Dr Oliver, a physician in practice, who conducted a series of experiments on his young son into whom he injected an extract of bovine suprarenal and detected a ‘definite narrowing of the radial artery’.1 The effect was confirmed in animals and led eventually to the isolation and synthesis of adrenaline/epinephrine in the early 1900s. Many related compounds were examined and, in 1910, Barger and Dale invented the word sympathomimetic2 and also pointed out that noradrenaline/norepinephrine mimicked the action of the sympathetic nervous system more closely than did adrenaline/epinephrine.
Classification of sympathomimetics
By mode of action
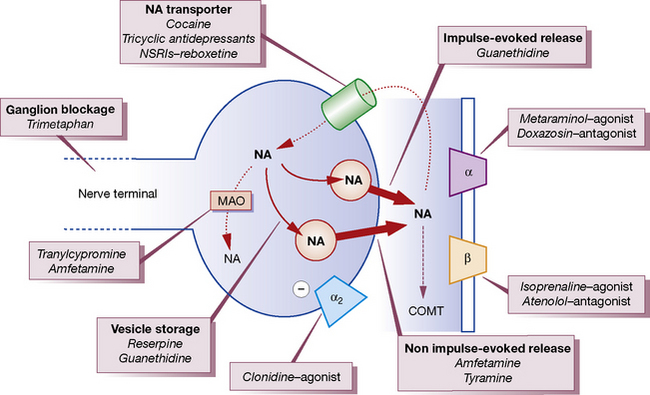
Noradrenaline/norepinephrine is synthesised and stored in vesicles within adrenergic nerve terminals (Fig. 23.1). The vesicles can be released from these stores by stimulating the nerve or by drugs (ephedrine, amfetamine). The noradrenaline/norepinephrine stores can also be replenished by intravenous infusion of noradrenaline/norepinephrine, and abolished by reserpine or by cutting the sympathetic nerve. Sympathomimetics may be classified on the basis of their sites of action (see Fig. 23.1) as acting:
1. Directly: adrenoceptor agonists, e.g. adrenaline/epinephrine, noradrenaline/norepinephrine, isoprenaline (isoproterenol), methoxamine, xylometazoline, metaraminol (entirely); and dopamine and phenylephrine (mainly).
2. Indirectly: by causing a release of preformed noradrenaline/norepinephrine from stores in nerve endings,3 e.g. amfetamines, tyramine; and ephedrine (largely).
3. By both mechanisms (1 and 2, though one usually predominates): other synthetic agents.
All of the above mechanisms operate in both the central and peripheral nervous systems, but discussion below will focus on agents that influence peripheral adrenergic mechanisms.
Tachyphylaxis
(rapidly diminishing response to repeated administration) is a particular feature of group 2 drugs. It reflects depletion of the ‘releasable’ pool of noradrenaline/norepinephrine from adrenergic nerve terminals that makes these agents less suitable as, for example, pressor agents than drugs in group 1. Longer-term tolerance (see p. 78) to the effects of direct sympathomimetics is much less of a clinical problem and reflects an alteration in adrenergic receptor density or coupling to second messenger systems.
Interactions of sympathomimetics
with other vasoactive drugs are complex. Some drugs block the reuptake mechanism for noradrenaline/norepinephrine in adrenergic nerve terminals and potentiate the pressor effects of noradrenaline/norepinephrine, e.g. cocaine, tricyclic antidepressants or highly noradrenaline/norepinephrine-selective reuptake inhibitors (NSRIs) such as reboxetine (see Fig. 23.1). Others deplete or destroy the intracellular stores within adrenergic nerve terminals (e.g. reserpine and guanethidine) and thus block the action of indirect sympathomimetics.
History
Subclassification of adrenoceptors is shown in Table 23.1.
Table 23.1 Clinically relevant aspects of adrenoceptor functions and actions of agonists
| α1-Adrenoceptor effectsa | β-Adrenoceptor effects |
|---|---|
| Eye:b mydriasis | |
| Heart (β1, β2):c | |
| increased rate (SA node) | |
| increased automaticity (AV node and muscle) | |
| increased velocity in conducting tissue | |
| increased contractility of myocardium | |
| increased oxygen consumption; decreased refractory period of all tissues | |
| Arterioles: | Arterioles: |
| constriction (only slight in coronary and cerebral) | dilatation (β2) |
| Bronchi (β2): relaxation | |
| Anti-inflammatory effect: | |
| inhibition of release of autacoids (histamine, leukotrienes) from mast cells, e.g. asthma in type I allergy | |
| Uterus: contraction (pregnant) | Uterus (β2): relaxation (pregnant) |
| Skeletal muscle: tremor (β2) | |
| Skin: sweat, pilomotor | |
| Male ejaculation | |
| Blood platelet: aggregation | |
| Metabolic effect: | Metabolic effects: |
| hyperkalaemia | hypokalaemia (β2) |
| hepatic glycogenolysis (β2) | |
| lipolysis (β1, β2) | |
| Bladder sphincter: contraction | Bladder detrusor: relaxation |
| Intestinal smooth muscle relaxation is mediated by α and β adrenoceptors. α2-adrenoceptor effects:a α2 receptors on the nerve ending, i.e. presynaptic autoreceptors, mediate negative feedback which inhibits noradrenaline/norepinephrine release | |
| Use of the term cardioselective to mean β1-receptor selective only, especially in the case of β-receptor blocking drugs, is no longer appropriate. | |
| Although in most species the β1 receptor is the only cardiac β receptor, this is not the case in humans. What is not generally appreciated is that the endogenous sympathetic neurotransmitter noradrenaline/norepinephrine has about a 20-fold selectivity for the β1 receptor – similar to that of the antagonist atenolol – with the consequence that under most circumstances, in most tissues, there is little or no β2-receptor stimulation to be affected by a non-selective β-blocker. Why asthmatics should be so sensitive to β-blockade is paradoxical: all the bronchial β receptors are β2, but the bronchi themselves are not innervated by noradrenergic fibres and the circulating adrenaline levels are, if anything, low in asthma. | |
a For the role of subtypes (α1 and α2), see prazosin.
b Effects on intraocular pressure involve both α and β adrenoceptors as well as cholinoceptors.
c Cardiac β1 receptors mediate effects of sympathetic nerve stimulation. Cardiac β2 receptors mediate effects of circulating adrenaline, when this is secreted at a sufficient rate, e.g. following myocardial infarction or in heart failure. Both receptors are coupled to the same intracellular signalling pathway (cyclic AMP production) and mediate the same biological effects.
Consequences of adrenoceptor activation
All adrenoceptors are members of the G-coupled family of receptor proteins, i.e. the receptor is coupled to its effector protein through special transduction proteins called G-proteins (themselves a large protein family). The effector protein differs among adrenoceptor subtypes. In the case of β-adrenoceptors, the effector is adenylyl cyclase and hence cyclic AMP is the second messenger molecule. For α-adrenoceptors, phospholipase C is the commonest effector protein and the second messenger here is inositol trisphosphate (IP3). It is the cascade of events initiated by the second messenger molecules that produces the variety of tissue effects shown in Table 23.1. Hence, specificity is provided by the receptor subtype, not the messengers.
Selectivity for adrenoceptors
The following classification of sympathomimetics and antagonists is based on selectivity for receptors and on use. But selectivity is relative, not absolute; some agonists act on both α and β receptors, some are partial agonists and, if sufficient drug is administered, many will extend their range. The same applies to selective antagonists (receptor blockers), e.g. a β1-selective-adrenoceptor blocker can cause severe exacerbation of asthma (a β2 effect), even at low dose. It is important to remember this because patients have died in the hands of doctors who have forgotten or been ignorant of it.4
Adrenoceptor agonists (see Table 23.1)
Effects of a sympathomimetic
To block all the effects of adrenaline/epinephrine and noradrenaline/norepinephrine, antagonists for both α and β receptors must be used. This can be a matter of practical importance, e.g. in phaeochromocytoma (see p. 419).
Physiological note
The termination of action of noradrenaline/norepinephrine released at nerve endings is by:
• reuptake into nerve endings by the noradrenaline/norepinephrine transporter where it is stored in vesicles or metabolised by monoamine oxidase (MAO) (see Fig. 23.1)
• diffusion away from the area of the nerve ending and the receptor (junctional cleft)
• metabolism (by extraneuronal MAO and catechol-O-methyltransferase, COMT).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree