Chapter 35 Adrenal corticosteroids, antagonists, corticotropin
• Adrenocortical steroids and their synthetic analogues.
• Inhibition of synthesis of adrenal steroids.
In 1855, Dr Thomas Addison, assisted in his observations by three colleagues, published his famous monograph ‘On the constitutional effects of disease on the suprarenal capsules’ (Addison’s disease). It was not until the late 1920s that the vital importance of the adrenal cortex was appreciated and the distinction made between the hormones secreted by the cortex and medulla.
Adrenal steroids and their synthetic analogues
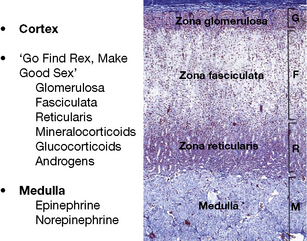
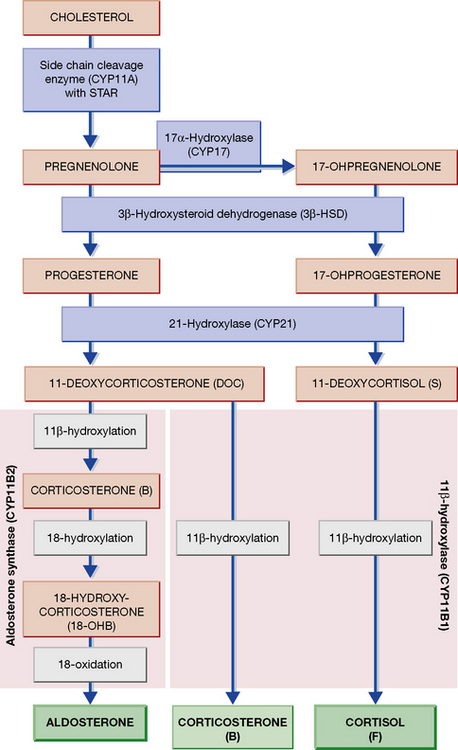
The adrenal is a composite endocrine gland, and each zone of the cortex synthesises a different predominant steroid; a mnemonic is offered in Figure 35.1, in which the first letter of each word is the first letter of each zone and its corresponding steroid product. The principal synthetic pathways are illustrated in Figure 35.2.
In the account that follows, the effects of hydrocortisone will be described and then other steroids in so far as they differ. In the context of this chapter, ‘adrenal steroid’ means a substance with hydrocortisone-like activity. Androgens are described in Chapter 38.
Mechanism of action
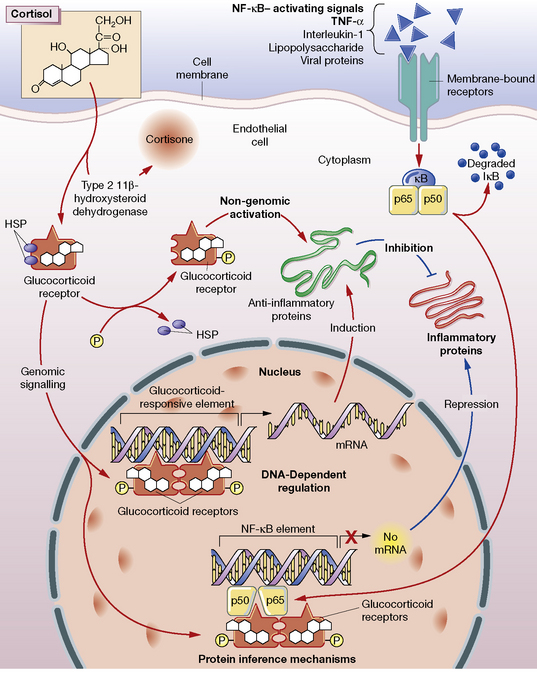
Glucocorticoids stimulate the cell through both a classical cytosolic receptor that, on binding with agonist, translocates to the nucleus, and an unidentified membrane-bound receptor (Fig. 35.3). The classical receptor is responsible for so-called genomic effects through either activation or repression of DNA transcription. Up-regulation of gene transcription occurs when the receptor dimerises on specific DNA glucocorticoid response elements (GREs) with consequent recruitment of coactivator proteins. Many of the undesired effects of glucocorticoid occur through this pathway.
Repression of DNA transcription occurs at slightly lower cortisol concentrations than required for transactivation. Through protein–protein interaction, the glucocorticoid–receptor complex inactivates pro-inflammatory transcription factors such as nuclear factor (NF)-κB and activator protein 1 (AP-1), preventing their stimulation of inflammatory mediators: prostaglandins, leukotrienes, cytokines and platelet-activating factor. These mediators would normally contribute to increased vascular permeability and subsequent changes including oedema, leucocyte migration and fibrin deposition.1
On organic metabolism
• Carbohydrate metabolism. Glycogenolysis and gluconeogenesis are increased and peripheral glucose utilisation is decreased (due to insulin antagonism).
• Protein metabolism. Anabolism (conversion of amino acids to protein) decreases but catabolism continues, so that there is a negative nitrogen balance with muscle wasting. The skin atrophies and this, with increased capillary fragility, causes bruising and striae. Healing of peptic ulcers or of wounds is delayed, as is fibrosis.
• Bone metabolism. Cortisol inhibits the number and function of osteoblasts, and the synthesis of collagen. Osteoporosis (reduction of bone protein matrix) is the main consequence of chronic glucocorticoid administration. Growth slows in children.
• Fat metabolism. Lipolysis is increased, and the secretion of leptin (the appetite suppressant) is inhibited. These actions lead to increased appetite and deposition of adipose tissue, particularly on shoulders, face and abdomen.
• Inflammatory response is depressed. Neutrophil and macrophage function are depressed, including the release of chemical mediators and the effects of these on capillaries.
• Allergic responses are suppressed. The antigen–antibody interaction is unaffected, but its injurious inflammatory consequences do not follow.
• Antibody production is lessened by heavy doses.
• Lymphoid tissue is reduced (including leukaemic lymphocytes).
• Renal excretion of urate is increased.
• Blood eosinophils reduce in number and neutrophils increase.
• Euphoria or psychotic states may occur, perhaps due to central nervous system (CNS) electrolyte changes.
• Anti-vitamin D action, see calciferol (p. 635).
• Reduction of hypercalcaemia, chiefly where this is due to excessive absorption of calcium from the gut (sarcoidosis, vitamin D intoxication).
• Urinary calcium excretion is increased and renal stones may form.
• Growth reduces where new cells are being added (growth in children), but not where they are replacing cells as in adult tissues.
• Suppression of hypothalamic–pituitary–adrenocortical feedback system (with delayed recovery) occurs with chronic use, so that abrupt withdrawal leaves the patient in a state of adrenocortical insufficiency.
Individual adrenal steroids
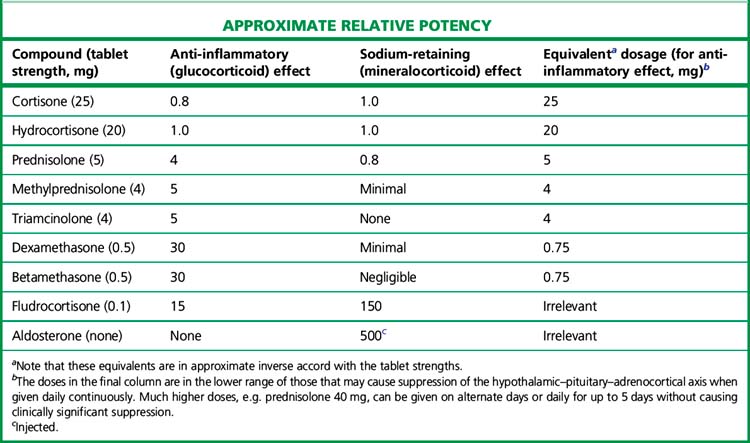
The relative potencies1 for glucocorticoid and mineralocorticoid (sodium-retaining) effects (Table 35.1) are central to the choice of agent in relation to clinical indication.
All drugs in Table 35.1 except aldosterone are active when swallowed, being protected from hepatic first-pass metabolism by high binding to plasma proteins. Some details of preparations and equivalent doses appear in the table. Injectable and topical forms are available (creams, suppositories, eye drops).
Fluorinated corticosteroids (triamcinolone, fludrocortisone)
Spironolactone
(see p. 550) is a competitive mineralocorticoid (aldosterone) antagonist. It is used in the treatment of primary hyperaldosteronism, as a diuretic in resistant hypertension, and when severe oedema is due to secondary hyperaldosteronism, e.g. cirrhosis, congestive cardiac failure. Long-term treatment increases survival in cardiac failure, possibly through blocking the fibrotic effect of aldosterone upon the heart. The dose of spironolactone is limited by its anti-androgen activity. Eplerenone has greater selectivity than spironolactone for the mineralocorticoid than androgen receptor, but has lower efficacy in blocking aldosterone and may need to be used together with amiloride.
Beclometasone, budesonide, fluticasone, mometasone and ciclesonide
are potent soluble steroids suitable for use by inhalation for asthma (see p. 474) and intranasally for hay fever. Patients swallow about 90% of an inhalation dose, which is then largely inactivated by hepatic first-pass. The drugs are listed in order of development; some newer agents possess properties (first-pass metabolism, high protein binding and lipophilicity) that may increase pulmonary residence time and reduce systemic effects. The main protection against these effects is simply that absorption through mouth, lungs and gut is low relative to the amounts used in systemic administration. The risk of suppression of the hypothalamic–pituitary–adrenal (HPA) axis is infrequent and dose dependent. The greatest risk of suppression appears to occur with high-dose fluticasone usage in children, who may present with hypoglycaemia and acute adrenal insufficiency.